BloodMatrix Contains Collagen Fibers, Calcium, and Phosphorus Salts — A Fortress of Structural Resilience
BloodMatrix Contains Collagen Fibers, Calcium, and Phosphorus Salts — A Fortress of Structural Resilience
The microscopic world within human blood plasma reveals a hidden blueprint of biological mastery: a complex matrix rich in collagen fibers, calcium ions, and phosphorus salts, converging to fortify tissues at the molecular level. This intricate composition not only supports vascular integrity but also exemplifies nature’s precision in building resilient frameworks essential for health. Far more than a passive fluid, blood plasma contains a dynamic scaffold where structural proteins and mineral deposits collaborate to maintain tissue strength, regulate mineral homeostasis, and enable rapid tissue repair.
The blood matrix’s central components — collagen fibers, calcium, and phosphorus — form a synergistic network that underpins connective tissue and bone structure. Collagen, the most abundant protein in the body, forms fibrous networks that provide tensile strength and flexibility. When combined with calcium and phosphorus in regulated proportions, these elements transform fluid into a functional, mineralized support system.
“Collagen provides the structural foundation, while calcium and phosphorus deliver the chemical reinforcement needed to withstand mechanical stress,” explains Dr. Elena Marquez, a biomedical researcher at the Institute for Matrix Biology. “This integration is vital for maintaining tissue elasticity and preventing degradation under daily physiological loads.”
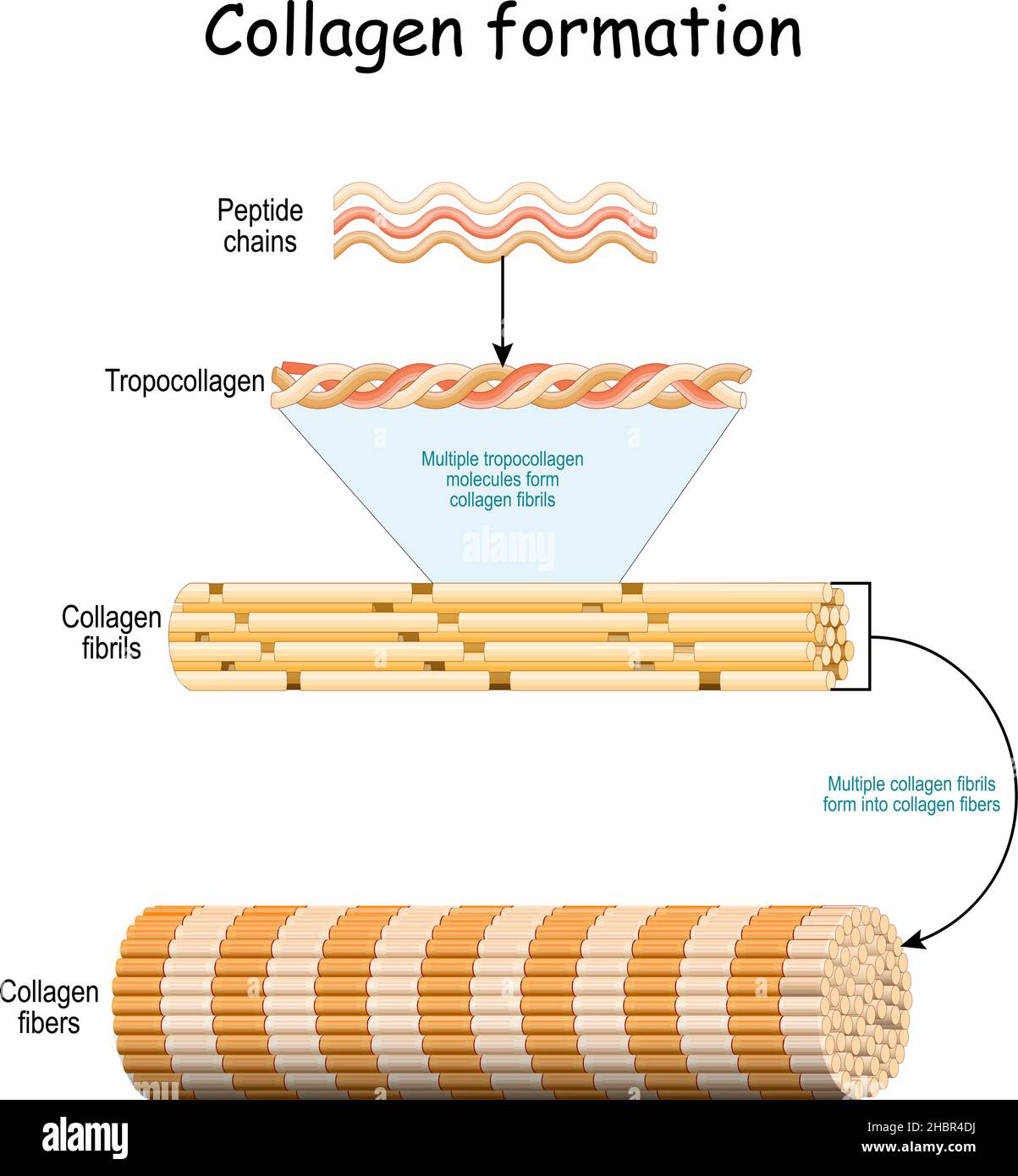
The Role of Collagen Fibers in Blood Matrix Architecture
Collagen fibers embedded within the blood matrix act as a durable scaffold, enabling cells to adhere, migrate, and organize efficiently.Predominantly Type I collagen, this structural protein forms continuous fibrillar networks that resist tensile forces and distribute mechanical stress across tissues. Their presence ensures vascular integrity, supporting the elastic recoil of blood vessels and preventing rupture under pressure. Moreover, collagen interacts directly with blood cells and matrix-bound enzymes, regulating clot formation and vascular repair processes.
In the matrix, collagen fibers do not operate in isolation; rather, they weave through a coordinated mineral environment, enhancing both flexibility and stability. Experts note that collagen’s structural role is complemented by its biochemical signaling capacity. For instance, specific collagen domains attract growth factors critical for angiogenesis — the formation of new blood vessels — and facilitate endothelial cell recruitment during wound healing.
“Collagen isn’t just passive scaffolding; it’s an active participant in tissue regeneration and homeostasis,” highlights Dr. Marco Lin, a tissue engineering specialist. “Its dynamic interaction with the surrounding mineral environment underscores its role in maintaining plasma’s functional versatility.”
Calcium and Phosphorus: Minéralising the Blood Scaffold
Calcium and phosphorus, though best known for their role in bone health, are fundamental to the functional integrity of the blood matrix.In plasma, these minerals exist primarily in ionic form — as Ca²⁺ and PO₄³⁻ — and serve dual purposes: supporting vascular smooth muscle contraction and enabling rapid mineralization where needed. They form transient complexes with matrix proteins, including collagen, helping to stabilize fiber networks while maintaining solubility to prevent pathological calcification. The ratio of calcium to phosphorus in plasma is tightly regulated, typically maintained around 2:1, ensuring optimal bioavailability without inducing tissue mineralization outside bony structures.
According to Dr. Aisha Rahman, a clinical chemist, “This balance allows calcium and phosphorus to reinforce the blood matrix without triggering calcification of soft tissues — a delicate equilibrium crucial for cardiovascular health.” When this balance falters, deviations can compromise vascular resilience and impair cellular signaling, leading to systemic dysfunction.
Beyond mineral homeostasis, calcium and phosphorus act as signaling molecules that influence matrix protein cross-linking and enzymatic activity.
For example, calcium ions activate enzymes like lysyl oxidase, which catalyzes the formation of covalent bonds between collagen and elastin fibers — strengthening the matrix at the biochemical level. Phosphorus, via its role in ATP and nucleotides, fuels cellular processes essential for ongoing tissue maintenance and recovery. The intricate dance between these mineral ions and collagen fibers exemplifies a finely tuned system designed for longevity and adaptability.
Functional Implications and Clinical Insights
The presence of collagen fibers alongside calcium and phosphorus salts in blood plasma has profound functional implications. It enhances tissue elasticity, supports wound healing, and enables rapid structural remodeling after injury. In aging and chronic diseases, disruptions in collagen integrity or mineral balance are linked to weakened vascular matrices, impaired tissue repair, and increased fragility — conditions observed in cardiovascular disorders and osteoporosis.Diagnostic advancements now leverage matrix components for early detection. For instance, elevated collagen breakdown products in plasma are emerging biomarkers of vascular damage, while altered calcium-phosphorus signaling is explored in metabolic bone-disease interfaces affecting blood stability. “Monitoring these matrix constituents provides insight into systemic health and early disease states,” says Dr.
Marquez. “They bridge the gap between structural biology and clinical medicine.”
Integrated Structural Dynamics: Collagen, Calcium, and Phosphorus as a Triad
The triad of collagen fibers, calcium, and phosphorus salts forms the cornerstone of blood matrix functionality — a sophisticated system where structural proteins and minerals co-evolved to maintain mechanical strength and biochemical responsiveness. Collagen fibers distribute stress and support cellular architecture; calcium and phosphorus provide controlled mineralization that reinforces matrix stability without rigidity.This synergy enables tissues to endure mechanical forces while remaining dynamically adaptable. Studies reveal that in healthy individuals, this integrated matrix facilitates efficient oxygen transport, rapid tissue repair, and sustained vascular tone. However, disturbances — such as aging, inflammation, or metabolic imbalances — can disrupt the collagen-mineral balance.
For example, in conditions like osteoarteriosclerosis, aberrant calcium-phosphorus deposition in vascular walls correlates with matrix stiffening and increased cardiovascular risk. “Maintaining this triad in optimal balance is essential,” emphasizes Dr. Lin.
“It’s not just about strength; it’s about flexibility, resilience, and long-term adaptability.”
Future Directions in Matrix Research
Ongoing research seeks to decode the full complexity of blood matrix interactions, particularly how collagen fibers dynamically respond to mineral fluctuations. Advanced imaging techniques and proteomic analyses are shedding light on the molecular interfaces between collagen fibrils and phosphate ions, revealing novel regulatory mechanisms. These insights promise breakthroughs in regenerative medicine, where engineered matrices mimicking natural collagen-mineral networks could enhance tissue engineering and vascular graft design.Moreover, understanding the role of calcium and phosphorus in regulating matrix stiffness is paving the way for targeted therapies. Drugs that modulate mineral solubility or collagen cross-linking may one day prevent or reverse age-related vascular decline, offering new hope for cardiovascular health. As scientists continue to unravel the navigation of this intricate biological system, the blood matrix emerges not just as a circulatory fluid but as a living, responsive network engineered for resilience.
Be it through enhancing tissue repair, guiding vascular function, or revealing early signs of disease, blood plasma’s collagen fibers, calcium, and phosphorus salts collectively form a cornerstone of human structural integrity—forged by evolution and central to life’s enduring complexity.



Related Post

Jodi Arias Case Exposed: Police Overlooked Blood-Stained Evidence in Trial Photos That Could Have Changed the Outcome

Metamorphosis Anime: Where Transformation Defines Destiny

Behind the Glamour: How Beauty Pageants Entrench Gender Bias Through Rigid Stereotypes and Inequality

Shoesland: Where Craftsmanship, Culture, and Comfort Converge in Footwear’s Evolution

