Bone Development Unveiled: Comparing Intramembranous and Endochondral Ossification in Human Skeletons
Bone Development Unveiled: Comparing Intramembranous and Endochondral Ossification in Human Skeletons
The human skeleton, a complex framework of rigid yet dynamic structures, owes its formation to two principal ossification processes: intramembranous and endochondral ossification. These mechanisms guide the transformation of cells into durable bone tissue, each operating through distinct biological pathways yet unified by their critical role in shaping the body’s structural foundation. Intramembranous ossification constructs flat bones such as the skull and clavicles from mesenchymal connective tissue, while endochondral ossification builds the majority of long bones—including the femur and humerus—starting from cartilage templates.
Understanding these contrasting processes reveals fundamental insights into skeletal development, physiology, and the evolutionary adaptability of human anatomy.
At the cellular level, intramembranous ossification begins when mesenchymal stem cells gather in connective tissue layers, forming osteoid—the initial unmineralized bone matrix. These progenitor cells differentiate into osteoblasts, which secrete collagen and minerals, laying down bone directly around collagen fibrils.
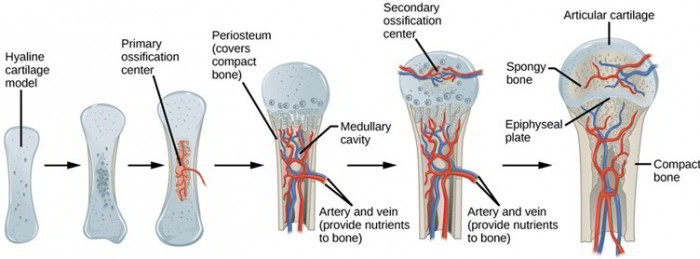
Unlike other capsullivan processes, this mechanism does not require a pre existing cartilage model—instead, bone forms uniquely within dense connective membranes. In contrast, endochondral ossification unfolds in stages within cartilaginous models, beginning with the formation of ossification centers in fetal development. Here, hyaline cartilage serves as the template; chondrocytes proliferate and later hypertrophy, secreting matrix that becomes calcified.
Blood vessels invade, bringing osteogenic progenitor cells that replace cartilage with bone via tightly regulated remodeling. “This substitution of cartilage by bone allows for longitudinal growth and the complex geometry of long bones,” explains Dr. Elena Torres, a developmental orthopedic biologist.
Definition and occurrence shape the functional divide between the two ossification types. Intramembranous ossification dominates in the flat cranial bones and the clavicle, forming through direct consolidation of mesenchymal assemblies. It is especially active during fetal development and remains crucial in cranial vault expansion throughout childhood and adolescence.
Endochondral ossification, by contrast, drives the formation of long bones and parts of the axial skeleton—including vertebrae and elements of the skull base—where lengthening and structural refinement are essential for movement and load-bearing. While both processes result in mineralized tissue, their dependency on preformed scaffolding versus de novo bone deposition distinguishes their roles in skeletal maturation. “These differences reflect evolutionary adaptations to distinct biomechanical demands,” notes Dr.
James Rhee, a leading scholar in comparative bone biology.
Structural outcomes differ significantly due to the unique mechanisms involved. Intramembranous ossification yields compact, dense bone with no growth plates—think of the thick assertive plates of the parietal skull bone.
This direct pathway favors rapid structural integrity but limits longitudinal bone growth. In contrast, endochondral ossification enables bones to elongate through the persistent activity of growth plates (epiphyseal plates), with chondrocytes continuously pushing height and width until skeletal maturity. The cartilage template provides a flexible, scalable scaffold that supports both growth and mechanical resilience, a balance vital for limb function and weight transmission.
Autopsy studies consistently show that over 80% of the skeletal framework develops via endochondral pathways, underscoring its centrality in postnatal skeletal maturation.
Key stages further distinguish the two ossification types. In intramembranous ossification, early osteoblast clusters secrete osteoid, followed by mineralization and vascular invasion forming trabeculae that fuse into a solid bone mass.
The process is synchronized with neural development, offering skull bones protective protection early in gestation. Endochondral ossification proceeds through distinct phases: initial cartilage formation, chondrocyte proliferation, matrix calcification, vascular invasion, osteoblast replacement, and finally bone remodeling. These stages—some taking years to complete—explain why growth plates are vulnerable to injury, and why disorders like achondroplasia directly disrupt this precise sequence.
Research estimates that disruptions in endochondral processes account for nearly 30% of pediatric bone development anomalies, highlighting their clinical relevance.
Mechanical and molecular drivers also vary between the two types. Intramembranous ossification depends on signaling molecules like BMPs (bone morphogenetic proteins) and Wnt pathways, which induce osteoblast differentiation within dense connective tissues.
Endochondral ossification hinges on a cascade involving Sox9 for cartilage formation, PTHrP to regulate chondrocyte proliferation and hypertrophy, and Indian hedgehog (Ihh) to initiate mineralization and osteoblast recruitment. The degradation of cartilage matrix is orchestrated by enzymes such as alkaline phosphatase and matrix metalloproteinases, tightly controlled to prevent premature degradation. This intricate biochemical dance ensures that bone replaces cartilage in a spatially and temporally precise manner.
Clinical implications of these processes extend into diagnostic and therapeutic realms. Radiographic and imaging technologies exploit differences in ossification timing and structure—X-rays detect early trabecular patterns from intramembranous sites, while growth plate evaluation via MRI or ultrasound is vital for monitoring endochondral development in children. Surgical interventions, such as corrective bone surgery or prosthetic implantation, often rely on understanding whether a bone defect lies within a direct-formed or cartilage-derived region.
Bone regeneration research further targets these pathways: stimulating BMP signaling accelerates intramembranous healing, while growth plate modulators offer potential treatments for growth disorders.
Ultimately, the dichotomy between intramembranous and endochondral ossification reveals the evolutionary precision embedded in human bone biology. While intramembranous ossification provides rapid, robust protection through direct skeletal assembly, endochondral ossification enables the dynamic adaptation and elongation essential for locomotion and mechanical support.
Their contrasting mechanisms, developmental timelines, structural outcomes, and molecular underpinnings highlight a dual system fine-tuned by millions of years of biological optimization. In the anatomy of the human skeleton, understanding these processes is not just academic—it is foundational to medicine, biomechanics, and human health.



Related Post
New York Time Zone Your Quick Guide: How to Master Local Time Zones in Daily Life

Mountain View, CA Zipcode 94043: The Pulse of Innovation, Home to Tech Giants and Urban Excellence

OSCOSC Foundation & SCSC Financing: Your Guide to Unlocking Strategic Capital in Emerging Markets

Lawyers Alliance for New York Empowers Legal Professionals with Unparalleled Advocacy and Support

