Charge of Carbon Atom: The Electron’s Shadow That Shapes Chemistry
Charge of Carbon Atom: The Electron’s Shadow That Shapes Chemistry
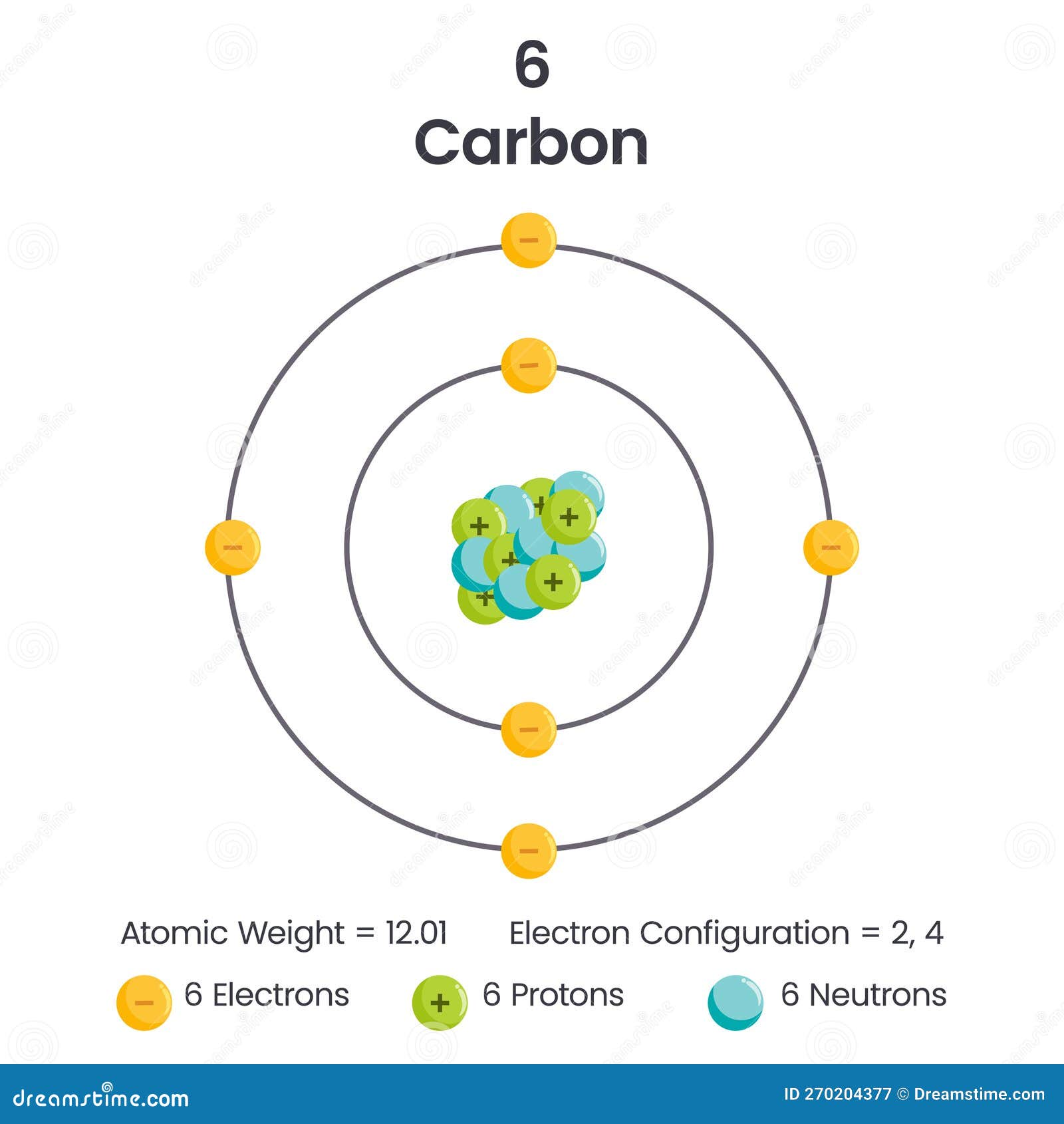
At the heart of every molecule lies a silent yet profound force: the charge of the carbon atom. With a valence electron configuration of 1s² 2s² 2p², carbon’s electronic behavior centers on its ability to carry a +4 formal charge when bonded to hydrogen, or to achieve neutrality through shared electrons in covalent bonds. This charge—both intrinsic and dynamic—dictates carbon’s role as the foundational element of organic chemistry, enabling the formation of complex structures from simple hydrocarbons to intricate biomolecules.
Understanding the charge of the carbon atom is essential to deciphering molecular interactions, reactivity patterns, and the architecture of life itself. Carbon’s atomic number is 6, placing it in the second period with two valence electrons. Its electronic shell structure limits bonding flexibility, yet this constraint fuels carbon’s unparalleled chemotaxis—the ability to bond selectively with four other atoms.
“Carbon is unique because it forms stable four-center bonds, a property rooted in its electronegativity and hybridization,” explains Dr. Elena Torres, a physical chemist specializing in molecular interactions. “The +4 formal charge emerges when carbon shares or donates electrons in ways that maximize stability, particularly in sp³ and sp² hybridized environments.” What defines the charge of the carbon atom?
It stems from electron distribution and bonding context. In methane (CH₄), carbon shares four electrons with hydrogen, achieving a full, neutral valence shell and no formal charge. Conversely, in carbon dioxide (CO₂), the carbon atom bears a +4 formal charge when viewed from an oxidation state perspective—though in reality, it shares electrons through double bonds, balancing charge through resonance.
“Carbon’s charge is context-dependent,” clarifies Professor Rajiv Mehta of the Department of Organic Chemistry at MIT. “It reflects localized electron density and how carbon interacts within a molecule—not an absolute charge unbound by environment.” This adaptability allows carbon to assume diverse oxidation states: from –4 in graphite phases to +4 in CO₂, illustrating the spectrum of its electron behavior. The –4 charge in carbides, for example, arises when carbon donates four electrons, stabilizing positively charged species.
In organic functional groups, carbon’s partial charge—often near-neutral but electronegatively biased—drives nucleophilic or electrophilic reactions. “A carbon center’s charge—whether full neutral, partial negative, or full +4—dictates its reactivity,” notes Dr. Lila Chen, an expert in reaction mechanisms.
“Finding these subtle shifts enables the precise design of catalysts, polymers, and pharmaceuticals.” Geochemically and biochemically, the charge of carbon atoms governs how they bind to surfaces, catalysts, or other atoms. In mineral surfaces critical for carbon sequestration, carbon’s +4 charge facilitates adsorption onto metal oxides. In enzymes, catalytic residues often involve carbon centers in transient charge states, lowering activation barriers.
Proteins rich in carbon—like collagen or hemoglobin—leverage carbon’s charge behavior for folding, stability, and molecular recognition. “Carbon’s duality—ability to share electrons or act as a hub—makes it irreplaceable,” asserts Dr. Amina Khalil, a synthetic biologist.
“Its charge characteristics are not static; they’re dynamic responses to the molecular environment.” Spectroscopic techniques reveal carbon’s charge nuances: Raman and infrared spectroscopy detect shifts in bonding environment linked to charge distribution. Nuclear Magnetic Resonance (NMR) further distinguishes cellular or reaction-specific charge states via shielding effects tied to electron density. These tools empower scientists to map carbon’s electron landscape with atomic precision.
In synthesis and sustainability, mastering the charge of carbon atom drives innovation. From designing carbon capture materials to engineering carbon-fixing enzymes, controlling electron distribution enables smarter chemistry. “The future of carbon utilization hinges on understanding and manipulating its charge—how it forms, shifts, and stabilizes,” says Mehta.
“The carbon atom’s charge isn’t just a number—it’s a blueprint for creation.” Ultimately, the charge of the carbon atom is far more than an atomic property—it’s a silent architect of molecular order, reactivity, and complexity. From life’s building blocks to advanced materials, its influence permeates every equation of chemistry, making it indispensable to scientific progress.
Atomic Structure and Bonding: The Foundation of Carbon’s Charge
Carbon’s electronic configuration—2s²


Related Post

Scoliosis Prefix and Suffix: Unlocking the Technical Language Behind Spinal Curvature Diagnoses
Internet Power Net: Your Ultimate Guide to Modern Connectivity and Unstoppable Power

Unblock Soccer Random Games: The Ultimate Unfiltered Experience
Purple Heads: The Unsung Cognitive Revolution Reshaping Sports Performance

