Decoding Fluorine’s Lewis Structures: How a Simple Atom Reveals Deep Chemical Insights
Decoding Fluorine’s Lewis Structures: How a Simple Atom Reveals Deep Chemical Insights
At the heart of modern chemistry lies a surprisingly powerful tool: the Lewis diagram. Nowhere is this more evident than in the case of fluorine — the most electronegative element, whose unique bonding behavior defies conventional expectations. The Fluorine Lewis Diagram is not merely a sketch; it is a revealing map of valence electrons, molecular connectivity, and reactivity patterns that shape the chemistry of life and materials alike.
By analyzing fluorine’s electron configuration and bonding logic, scientists decode how this tiny atom orchestrates complex chemical interactions, influencing everything from pharmaceutical design to industrial catalysts.
The Fluorine Lewis Diagram: A Window into Electron Behavior
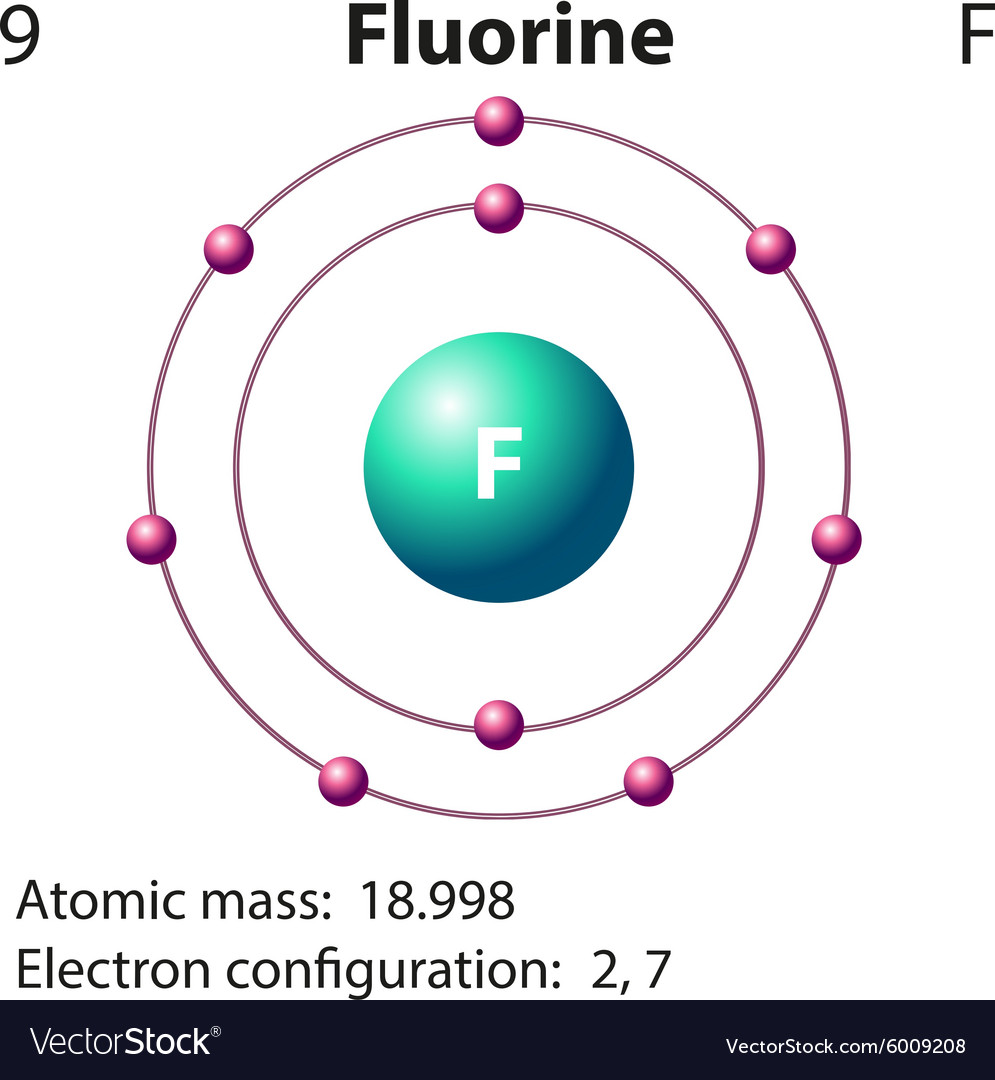
Fluorine, with atomic number 9 and electron configuration 1s² 2s² 2p⁵, occupies a special place in the periodic table. Its valence shell holds seven electrons, making it only one electron short of achieving a stable noble gas configuration.This inherent electron deficiency drives fluorine’s distinctive chemistry. The Lewis structure for fluorine—though it typically appears as a monatomic atom—offers a foundational insight into its bonding strategies. Since fluorine rarely forms molecules as a lone atom (it tends to exist diatomically, F₂), its Lewis diagrams focus on interactions with other elements.
In every compound, fluorine’s electrons are redistributed to achieve full octets, emphasizing its strong electronegativity and reactivity.
| Atom | Fluorine (F) | Electron count (valence shell) | 7 electrons | Electronegativity (Pauling scale) | 4.0 |
|---|---|---|---|---|---|
| Bonding | Uses shared or transferred electrons to complete octet | max 8 valence electrons in bonded species | forms single, double, or higher-order bonds in compounds | ||
| Key Feature | The empty p-orbital availability allows fluorine to accept one electron and stabilize in compounds like hydrofluoric acid (HF) or organofluorine pharmaceuticals | ||||
| Fluorine atoms in drug molecules enhance metabolic stability, lipophilicity, and binding affinity. Common drugs like Prozac (fluoxetine) and Keytruda (pembrolizumab) rely on fluorinated moieties to achieve targeted action. | Fluorine’s Lewis-driven electron density affects pKa and pI, critical parameters in drug absorption and distribution. | Examples include antivir
Related PostOpen Season: Scared Silly Transcript – Full Dialogue Guide to the Wildly Hilarious Moments
Roblox Festival Com: The Pivotal Celebration Bringing Creativity, Community, and Craft to a Global Audience
Boost Your English Writing With Systematic Ispoken Practice
G Michael Hopf’s Religious Views Explained: A Deep Dive into Faith, Reason, and Moral Framework
When Is Sza’s Album Coming Out? The Wait is Ending—Here’s Everything You Need to Know |
