Decoding Nitrogen’s Odd Trio: Lewis Dot Structures Reveal Hidden Chemistry of Nitrogen 3
Decoding Nitrogen’s Odd Trio: Lewis Dot Structures Reveal Hidden Chemistry of Nitrogen 3
In the unpredictable realm of molecular bonding, nitrogen 3 — represented by the symbolic formula N₃ — stands as a quiet enigma in chemistry’s tapestry. At first glance, N₃ may appear as nothing more than a linear molecule with only three nitrogen atoms linked together, yet its electronic architecture unveils a complexity that shapes its reactivity and scientific intrigue. Applying Lewis dot structure principles unlocks a precise visualization of electrons, revealing how this small but significant diatomic arrangement governs nitrogen’s elusive behavior in molecular systems.
Understanding N₃ demands careful scrutiny beyond simple bonding models. Unlike stable nitrogen molecules such as N₂, N₃ exists primarily as a high-energy intermediate or transient species under specific reaction conditions. Its formation and stability hinge on subtle electronic interactions that challenge classical bonding theories.
By analyzing its Lewis dot structure, chemists gain essential insight into valence electron distribution, bond formation, and energetic feasibility.
Electron Count and Structural Foundations of N₃
N₃ consists of three nitrogen atoms, each contributing five valence electrons per atom—totaling fifteen electrons across the molecule. To build a plausible Lewis structure, these electrons must be arranged to satisfy the octet rule while minimizing formal charges and maximizing stability.However, nitrogen’s capability to exceed an octet—through expanded valence shells enabled by d-orbitals—complicates straightforward octet adherence. Using the core principle of Lewis dot structures, each nitrogen atom must achieve a stable electron configuration; nitrogen typically seeks eight electrons, but in extended systems like N₃, dispersed electron density allows flexibility. The linear geometry of N₃—N—N—N— reflects its planar alignment, positioning atoms for optimal p-orbital overlap and σ-bond formation.
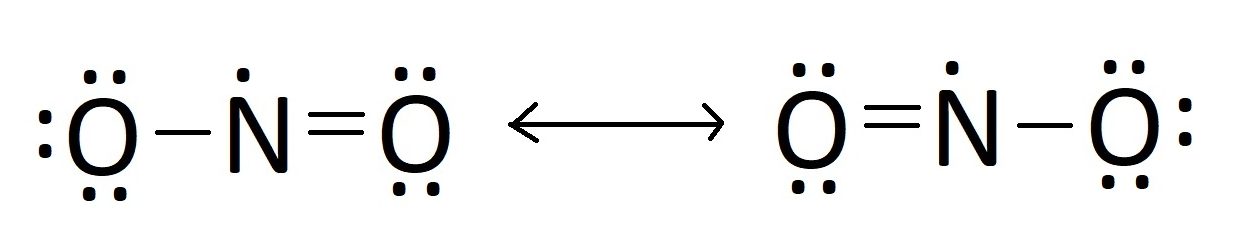
While nitrogen atoms could share three single bonds (N–N–N), the actual Lewis structure reveals distributed electron density, often illustrated with resonance-like contributions. In reality, the bonding is not fixed but involves electron delocalization across the entire trimolecular unit, stabilized through partial double-bond character and hyperconjugative interactions. Resonance and Electron Delocalization in



Related Post

Revolutionizing Digital Collaboration: How 9Xbuddy is Transforming Team Productivity Across Industries

Stephen King’s Wife: The Quiet Strength Behind the Horror Legend’s Success

Why Millions Are Slouching Toward Bethlehem—a City Steeped in History and Spiritual Yearning

Shin Black Ramen A Deep Dive: Unweaving the Myth, Merging Tradition, and Reinventing Flavor

