Decoding the Oxygen Atom: How Lewis Dot Structure Reveals the Heart of Chemical Reactivity
Decoding the Oxygen Atom: How Lewis Dot Structure Reveals the Heart of Chemical Reactivity
At the core of molecular chemistry lies a silent architect—an invisible blueprint that determines how oxygen—arguably the most vital element for life—interacts with the world. The Lewis Dot Structure for oxygen, though deceptively simple, offers profound insight into its electronic nature, bonding behavior, and reactivity. By analyzing oxygen’s electron arrangement through this foundational model, chemists unlock the mechanisms behind everything from cellular respiration to water’s extraordinary properties.
Far more than just a drawing of dots and lines, this structure reveals the silent forces driving chemical transformations, making it indispensable for understanding both benign and hazardous molecular interactions. The Lewis Dot Structure for oxygen represents the atom’s valence electrons in a visual and chemically meaningful way. Oxygen, with an atomic number of 8, possesses six valence electrons—two in its outer shell, shown as two dots surrounding the atomic symbol.
Unlike more complex molecules, oxygen typically exists as O before forming bonds; in its elemental form, it does not bear a full negative charge but maintains a neutral electronic configuration. When drawing Lewis structures, these six dots are the starting point—arranged to illustrate sharing, pairing, and potential reactivity.
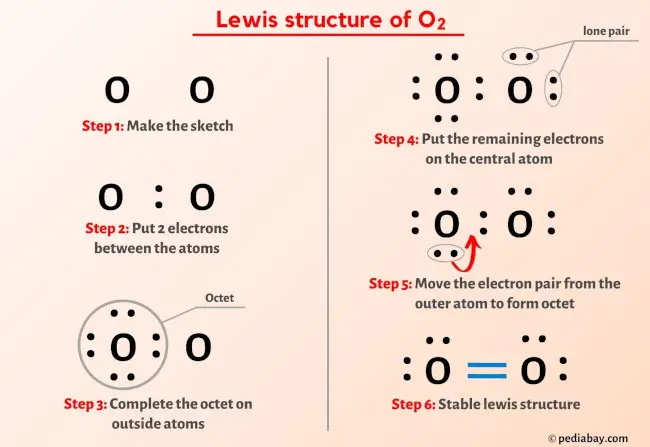
Central to oxygen’s chemical behavior is its tendency to complete a stable octet, adhering to the octet rule—a principle stating that atoms strive for eight electrons in their valence shell for maximum stability.
In its free symbol form, oxygen displays six isolated electrons, a configuration that signals a desire to bond. This reactivity is evident when oxygen forms diatomic molecules (O₂), though that specific pairing involves a resonance structure rather than a static Lewis dot diagram—highlighting both simplicity and complexity in representation. In bonding scenarios, future structures will show oxygen sharing electrons to satisfy this octet, whether through single, double, or even triangle-like arrangements influenced by hybridization.
The Structural Logic of Oxygen’s Electron Configuration
This arrangement captures oxygen’s electron deficiency under standard conditions, explaining its polar nature and preference for forming bonds. When oxygen participates in bonding, Lewis structures evolve to show shared electron pairs. In molecular oxygen (O₂), a resonance hybrid dominates despite the breakdown of a single static dot depiction—oxygen forms a double bond composed of two shared pairs, but with an alternate representation including unpaired electrons, reflecting its paramagnetic properties.
Premise: Lewis Structures and Chemical Connectivity
Each dot in the Lewis depiction carries meaning: unpaired electrons indicate potential for radical reactions, lone pairs influence molecular polarity, and bond pair counts determine bond order and strength.
These visual cues enable rapid analysis of how oxygen interacts with hydrogen, halogens, and metals. For example, in hydrogen peroxide (H₂O₂), the Lewis structure reveals two pendant hydroxyl groups each bearing lone pairs, enhancing nucleophilicity and reactivity in organic oxidation processes. The precision of these electron maps ensures chemists can anticipate environmental impacts—from atmospheric ozone formation to industrial oxidant applications—based on oxygen’s fundamental behavior as modeled by Lewis theory.
Beyond Static Icons: Dynamic Implications of the Lewis Model
These scenarios underscore the structure’s utility in predicting stability, charge distribution, and bond lifetimes. Furthermore, modern applications extend beyond pure theory. In synthetic chemistry, engineers design catalysts and reagents with atomic precision, using Lewis principles to tailor oxygen’s reactivity.
For instance, metal-organic frameworks (MOFs) leverage oxygen’s affinity to form porous structures ideal for gas storage and separation. Even environmental science relies on this model: tracing oxygen’s journey through biological and chemical cycles depends on accurate representations of its bonding behavior.
The power of the Lewis Dot Structure for oxygen lies in its ability to condense quantum complexity into an accessible visual language—bridging electron migration and macroscopic outcomes.
It transforms abstract quantum states into familiar icons of pairing, charge, and geometry, empowering students, researchers, and industry professionals alike.
Ultimately, the Lewis Dot Structure for oxygen is not merely a pedagogical tool but a cornerstone of chemical reasoning. From classroom diagrams to industrial process design, this model endures as a testament to the elegance of simplicity in explaining nature’s most reactive element. Its clarity reveals not just lines and dots, but the dynamic heartbeat of chemistry—where every unpaired dot is a potential reaction, and every shared pair paves a path forward.


Related Post

How to Catch Every Moment: The Jackson Hole Rodeo Seating Chart Revolutionizes Viewer Experience

A042M FRP Unlock: The Ultimate Test Point Guide & Tool Insights for A042M FRP Systems

Blue Jays Managers and Coaches: A Complete History of Strategic Leadership

What Is The Time of Texas? Unpacking the State’s Complex Time Zone Reality

