Hydrostatic vs Osmotic Pressure: The Invisible Battle Shaping Life at the Cellular Level
Hydrostatic vs Osmotic Pressure: The Invisible Battle Shaping Life at the Cellular Level
At the microscopic frontier of biology and physics, two fundamental forces—the hydrostatic and osmotic pressures—silently govern fluid dynamics across cell membranes, sustaining life and dictating cellular behavior. Though distinct in origin, these opposing pressures entwine in a delicate equilibrium that determines everything from kidney function to plant turgor and the efficacy of medical therapies. Understanding their interplay reveals not only the elegance of nature’s design but also the critical implications for human health and biotechnology.
Defining the Forces: Hydrostatic Pressure Explained
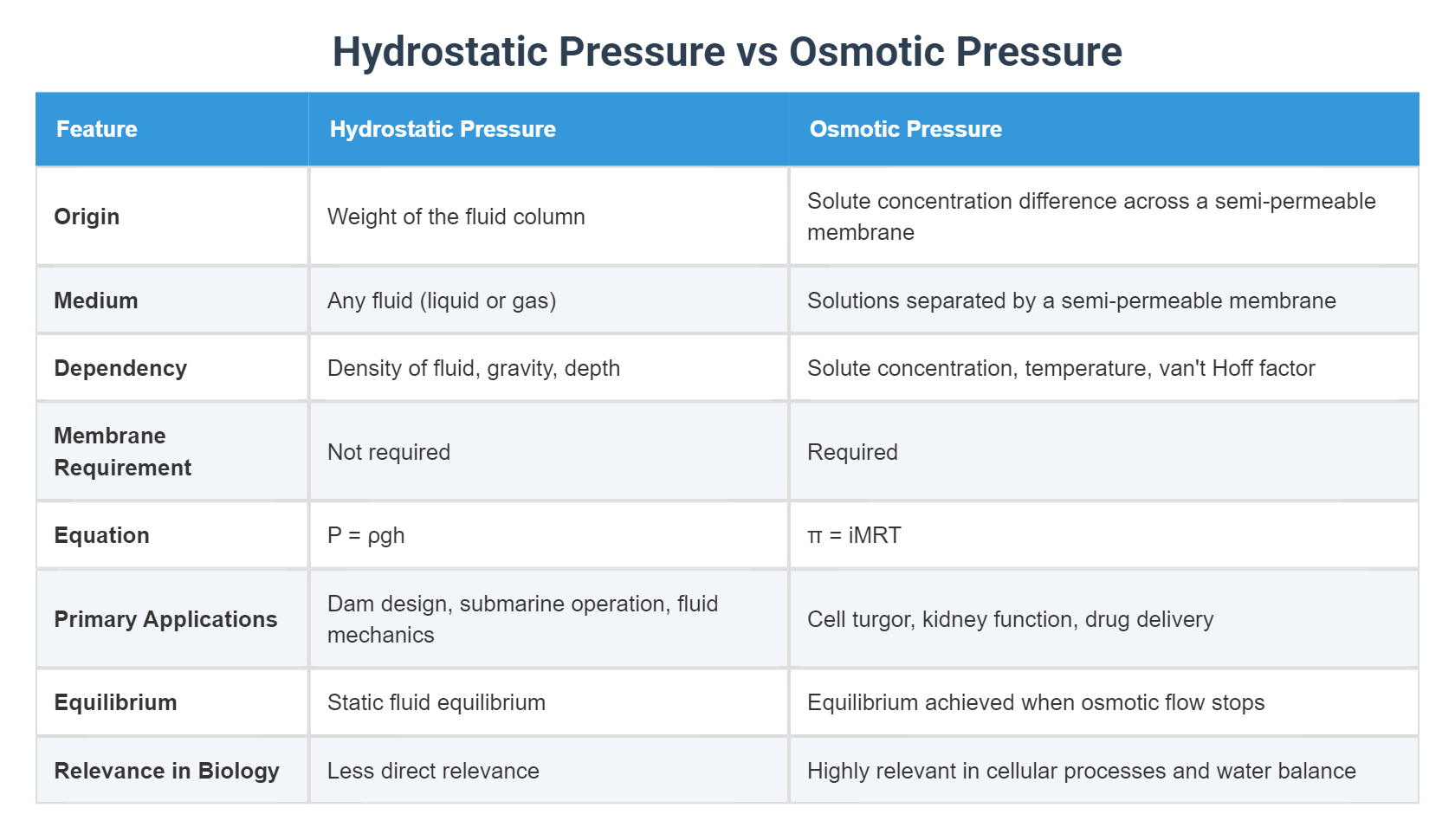
Hydrostatic pressure arises from the weight of a fluid column or external fluid force, a principle entrenched in classical fluid mechanics. “Hydrostatic pressure is the pressure exerted by a fluid at equilibrium due to the force of gravity,” explains Dr. Elena Torres, a biophysicist at MIT.“It increases with depth, following the familiar equation P = ρgh, where ρ is fluid density, g is gravitational acceleration, and h is height of the fluid column.” This pressure governs fluid behavior in blood vessels, soil pores, and ocean depths. For example, in microcirculation, hydrostatic pressure pushes fluid from capillaries into tissues, enabling nutrient exchange. Yet, it alone cannot dictate fluid movement across membranes—this is where osmotic pressure takes center stage.
< spend several paragraphs exploring osmotic pressure, its molecular basis, and regulatory role> Osmotic pressure, in contrast, is a solution-diffusion phenomenon driven by concentration gradients, not gravity. It arises when a semipermeable membrane separates solutions of differing solute concentrations, compelling solvent molecules—typically water—to migrate toward the higher solute side. “Osmotic pressure is the force required to prevent water from flowing across a selectively permeable membrane,” clarifies Dr.
Marcus Lin, a cellular physiologist. “It is quantified by van’t Hoff’s law: π = iCRT, where π is osmotic pressure, i is the van’t Hoff factor, C is molar concentration, R is the gas constant, and T is absolute temperature.” This pressure counters excessive fluid loss or gain in cells, preserving structure and function. For instance, in red blood cells, osmotic balance ensures they neither shrink (crenation) nor burst (hemolysis), maintaining circulation efficiency.
< structured breakdown of key differences and practical contexts> The essential distinction lies in their triggers and regulatory domains: - **Origin**: Hydrostatic pressure stems from gravity or mechanical force; osmotic pressure emerges from solute concentration differences across membranes. - **Role in Cells**: Hydrostatic pressure influences fluid movement in bulk flow systems like blood and lymph; osmotic pressure stabilizes cell volume by governing water flux. - **Physiological Governors**: Hydrostatic systems dominate in large circulatory and environmental flows, while osmotic pressure governs intracellular and renal homeostasis.
- **Mathematical Models**: Hydrostatic pressure follows hydrostatic equations; osmotic pressure adheres to colligative properties via van’t Hoff’s equation. These differences manifest in critical biological contexts. In the human kidney, osmotic pressure regulates urine concentration by balancing water reabsorption in nephrons, while hydrostatic pressure drives fluid filtration at glomeruli.
In plants, osmotic pressure sustains turgor in guard cells, enabling stomatal opening and closing—vital for gas exchange and drought response. Yet, an imbalance, such as in edematous tissues where hydrostatic pressure rises abnormally, can cause cellular swelling and dysfunction, underscoring the necessity of precision. < practical implications and medical frontiers> The clinical relevance of these pressures is profound.
In oncology, intraoperative tumor hydration manipulates hydrostatic gradients to improve drug delivery. In neurocritical care, managing intracranial pressure demands a fine understanding of both pressures to prevent cerebral edema or collapse. Dialysis machines exploit osmotic principles by using semipermeable membranes to remove waste from blood, mimicking the kidney’s natural osmotic regulation.
Researchers are also pushing boundaries in drug delivery and tissue engineering, designing nanoparticles and hydrogels that exploit osmotic pressure for controlled release, while engineers study microfluidic systems where hydrostatic forces shape lab-on-a-chip environments. “We’re learning to mimic nature’s fluid logic,” says Dr. Lin.
“By mastering these pressures, we unlock new ways to treat disease and sustain life.”
Future Insights: Beyond Nature into Innovation
As computational models grow more sophisticated, scientists now simulate hydrostatic and osmotic pressures at molecular scales, revealing hidden dynamics in nanopores and biological channels. Innovations such as osmotic power generators—harnessing salinity gradients to produce electricity—demonstrate how deepening our understanding of these forces can drive sustainable technology. While hydrostatic pressure governs flow on macroscopic scales, osmotic pressure quietly orchestrates life at the cellular threshold.Together, these pressures form an unseen yet omnipresent network—each a silent architect of physiological balance. Their precise interplay sustains cells, organs, and ecosystems alike. In mastering this microscopic tug-of-war, humanity not only deepens its grasp of biology but also pioneers solutions that span medicine, energy, and environmental resilience.
Hydrostatic and osmotic pressures, though conceptually distinct, are two halves of a balanced equation—one rooted in force and gravity, the other in solute concentration and diffusion. Their convergence defines the微观 world where life thrives.



Related Post

Billie Eilish’s Nip Slip Controversy: How a Fashion Statement Sparked Global Dialogue and Redefined Pop Culture Norms

Spy X Family: Is the Manga Finally Finished or Still in Suspense?

Exploring The Life Of Aunjanue Ellis And Her Husband: From Broadway To Hollywood Romance

Top Petroleum Engineering Schools: Where Future Energy Leaders Are Built

