Mastering Chemical Equilibrium with the BalancingReactionsCalculator: Precision in Reaction Balance
Mastering Chemical Equilibrium with the BalancingReactionsCalculator: Precision in Reaction Balance
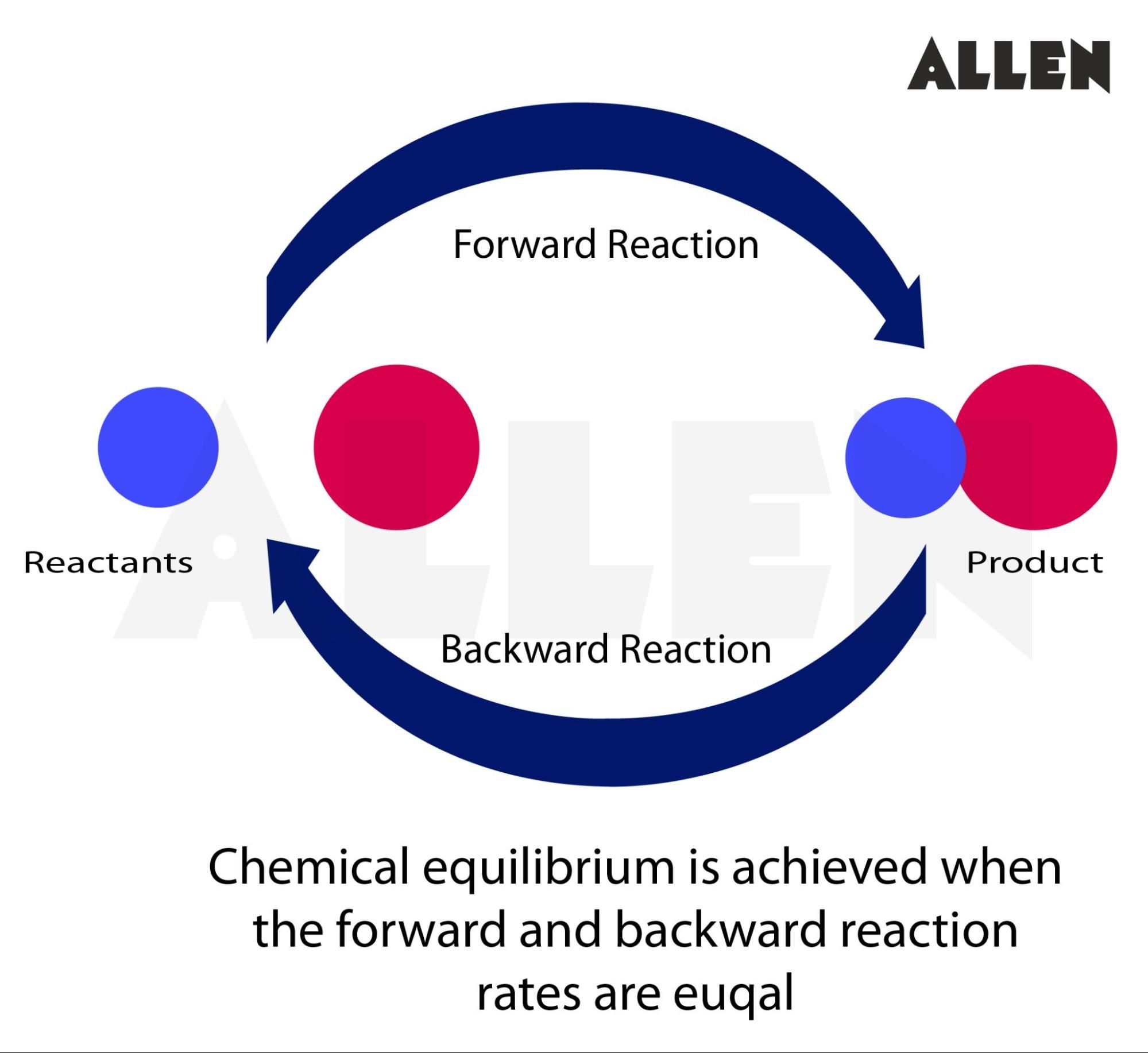
Understanding chemical reactions at equilibrium is fundamental to chemistry—and achieving precise balance calculations remains one of the most critical challenges for students, researchers, and industrial chemists alike. The BalancingReactionsCalculator emerges as a powerful tool, enabling users to navigate complex stoichiometric challenges with accuracy and speed. By transforming abstract reaction equations into balanced, quantifiable forms, this calculator illuminates the intricate dance of molecules as they reach dynamic equilibrium.
The BalancingReactionsCalculator is more than a mechanical solution—it is a bridge between theoretical principles and practical application. Designed with both accuracy and user accessibility in mind, it simplifies the traditionally time-consuming process of balancing chemical equations, especially for multi-reactant, multi-product systems. Whether calculating the effects of concentration shifts, temperature changes, or pressure variations, the tool supports detailed reaction analysis grounded in Le Chatelier’s Principle and mass conservation.
#### Core Capabilities of the BalancingReactionsCalculator At its core, the calculator supports several key functionalities: - Automated balancing of elementary and compound reactions across binary, ternary, and higher-order stoichiometries. - Visualization of reaction coefficients and molecular transformations, enhancing conceptual understanding. - Integration with equilibrium constants (Kₐ, Kᵇ, K_c, K_p) to compute shift predictions under varying conditions.
- Dynamic adjustment of variables—such as initial concentrations or temperature—to simulate real-world scenarios. “What sets this apart is its ability to not only balance equations but contextualize them within equilibrium frameworks,” says Dr. Elena Martinez, a physical chemist specializing in reaction dynamics.
“Users gain immediate insight into how ratios of reactants and products respond to perturbations—essential for lab work, process design, and environmental modeling.” #### How It Transforms Reaction Analysis Balancing chemical equations manually demands not only mathematical fluency but also a deep grasp of mole ratios and conservation laws. The BalancingReactionsCalculator streamlines this process through algorithmic precision, reducing human error while preserving educational value. Users input raw reaction data—numbered coefficients, molecular formulas—and the tool produces a balanced equation alongside quantifiable mole relationships.
For example, consider the acid dissociation of acetic acid: HC₂H₃O₂ ⇌ H⁺ + C₂H₃O₂⁻ The calculator confirms the coefficient rearrangement: HC₂H₃O₂ ⇌ 1H⁺ + 1C₂H₃O₂⁻ but goes further by enabling inquiry into equilibrium shifts. If sodium acetate is added, increasing C₂H₃O₂⁻ concentration, the tool instantly recalculates required forward reaction rates to reestablish balance—empowering students and professionals to predict system responses with confidence. Beyond balancing,



Related Post

Coco Gauff’s Parents: The Unseen Blueprint Behind a Rising-Star’s Trailblazing Journey

Xo Kitty Videos: The Viral Phenomenon Redefining Online Comedy and Creativity

The Power of Algebra in Action: Mastering Glencoe Algebra 2

Unlock Secure Naval Access: Mastering the www.Navyfcu.Org Login Experience