S Valence Electrons: The Hidden Forces Shaping Chemical Identity
S Valence Electrons: The Hidden Forces Shaping Chemical Identity
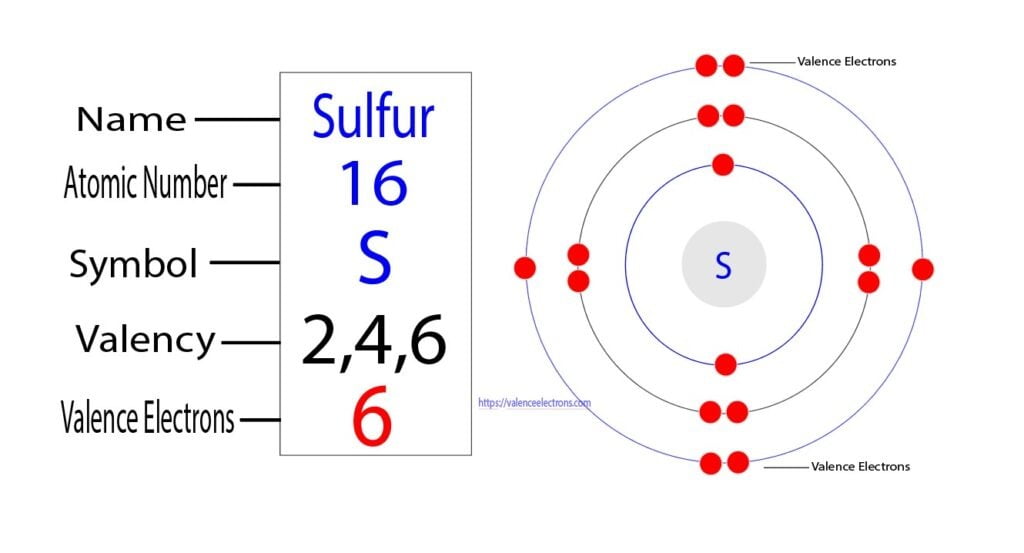
At the core of every atom’s reactivity lies a microscopic ballet of electrons—specifically, the S valence electrons. These outermost electrons determine how atoms bond, interact, and transform, forming the foundation of chemistry’s predictive power. Unlike inner-shell electrons, which remain passive, valence electrons engage directly with other atoms, driving processes critical to life and materials science.
With their delicate balance of stability and reactivity, S valence electrons govern everything from the synthesis of life’s building blocks to the performance of advanced industrial catalysts.
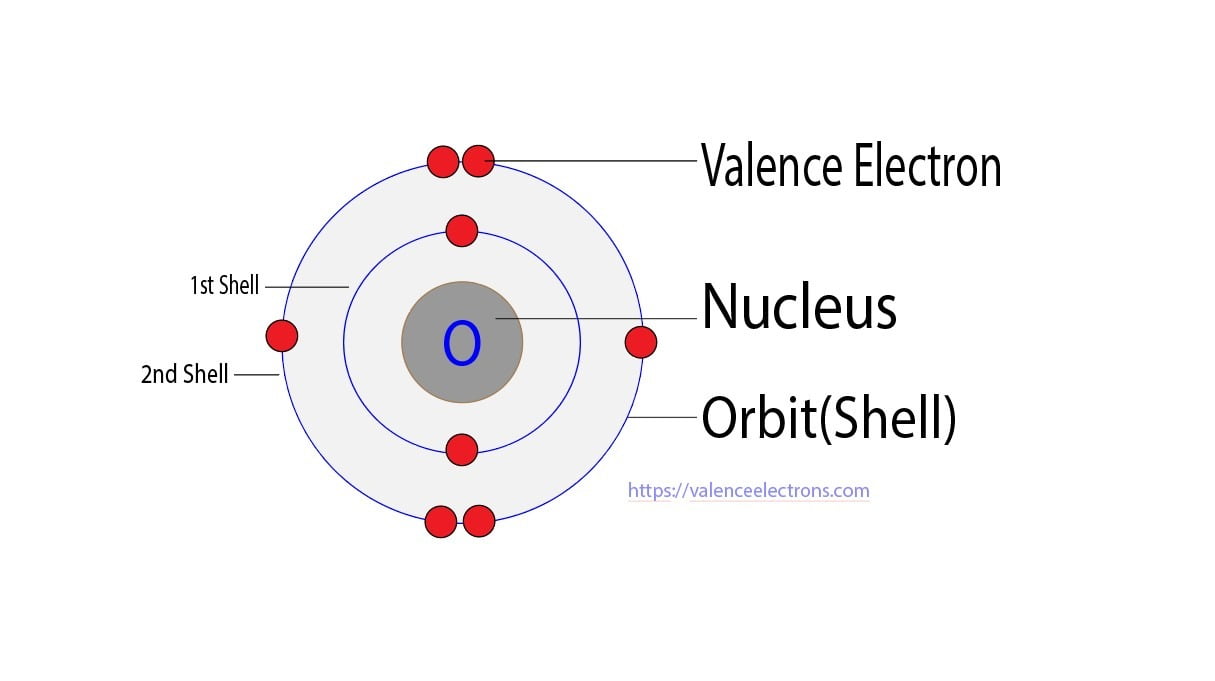
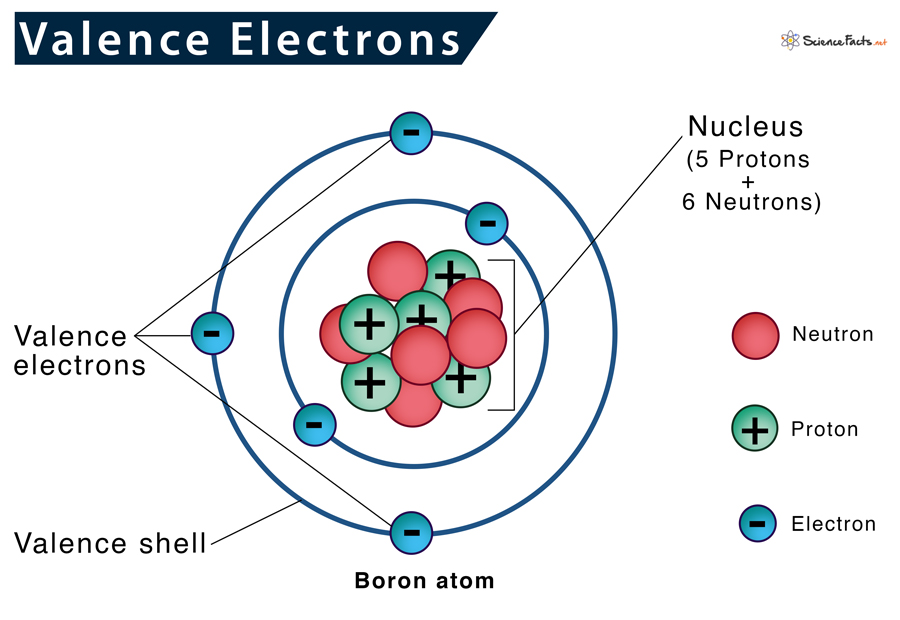
Valence electrons are defined as the electrons occupying the highest energy level of an atom, the outermost shell. For main-group elements, these determine chemical class, bonding behavior, and placement in the periodic table.
In
Related Post
The Strategic Blueprint of Organizational Resilience: How Rutrecker’s Framework Transforms Operational Agility and Sustained Success

Watch CTV Live Stream Online Free: Your Ultimate Guide to Seamless, Ad-Free Broadcast Access

Bisonville Football Your Ultimate Fan Forum Unleashed: The Heartbeat of Community on the Gridiron

Jacqui Heinrich Husband: From Soaring Pop Stardom to Resilient Matriarchal Voice

