SN1 vs SN2: The Chemical Dance of Substitution Reactions
SN1 vs SN2: The Chemical Dance of Substitution Reactions
Organic chemistry’s battle between SN1 and SN2 reactions defines how alkyl halides transform into alcohols and other functional groups, with each pathway offering distinct strengths, limitations, and mechanistic pathways. While both reaction types achieve nucleophilic substitution—replacing a leaving group with an incoming nucleophile—they diverge sharply in kinetics, stereochemistry, and selectivity. Understanding their differences is essential for predicting reaction outcomes and designing efficient synthetic routes.
SN1 and SN2 reactions represent two fundamental strategies in substitution chemistry.The SN1 mechanism proceeds through a two-step process: first, the loss of the leaving group generates a carbocation intermediate; second, the nucleophile attacks from either face, often resulting in racemization. In contrast, SN2 is a single-step concerted reaction where the nucleophile attacks the electrophilic carbon as the leaving group departs, leading to a stereochemically inverted product. The choice between these pathways shapes everything from reaction conditions to product purity, making their comparison not just academically relevant but industrially critical.
Mechanistic Foundations: Stepwise vs. Concerted Reactions
At the heart of SN1 and SN2 lies a fundamental difference in mechanism that governs their behavior. The SN1 pathway relies on heterolytic cleavage of the carbon-leaving group bond, forming a planar carbocation—an intermediate stabilized by resonance in tertiary substrates but prone to rearrangements.“The slow, rate-determining first step means SN1 reactions are unimolecular, with rate dependent only on substrate concentration,” explains chemist Dr. Elena Markov in a 2022 study published in *Organic Reactions*. “This makes SN1 highly sensitive to carbocation stability and solvent effects.” The SN2 mechanism, in contrast, is bimolecular and stereospecific: as the nucleophile approaches, the outgoing leaving group exits in a single concerted step.
“In SN2, inversion of configuration—known as Walden inversion—is a hallmark feature,” notes Mark Smith, professor of organic chemistry at MIT. “This requires strong nucleophiles, relatively unhindered substrates, and a reaction that avoids steric congestion.” Because SN1 and SN2 progress along entirely different mechanistic terrains, selecting the right pathway dictates not only product identity but also reaction reliability and selectivity.
Substrate Reactivity: The Role of Tertiary, Secondary, and Primary Halides
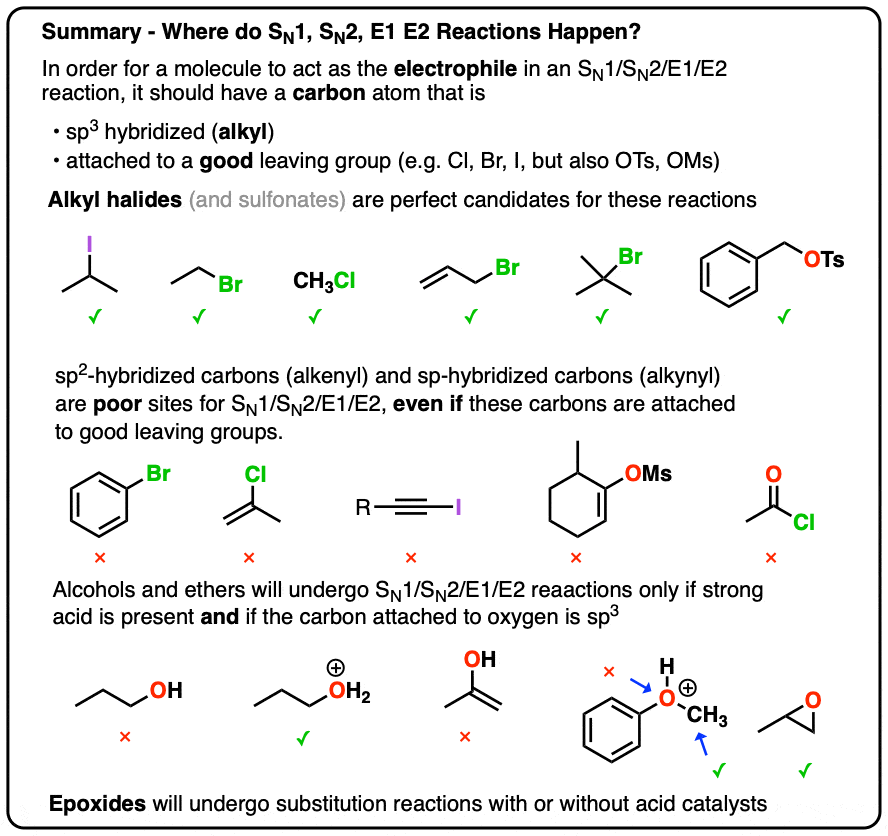
Substrate structure profoundly influences which mechanism dominates, making substrate classification a cornerstone of predicting reaction behavior.Tertiary alkyl halides overwhelmingly favor SN1 due to carbocation stability—three adjacent carbons stabilize the positive charge through hyperconjugation and inductive effects. “Tertiary substrates are practically mandatory for SN1,” confirms Dr. Lila Chen, a leading authority in reaction mechanisms, “since the energy barrier for carbocation formation is offset by the stability of the intermediate.” Secondary halides exhibit greater mechanistic flexibility; they can undergo either SN1 or SN2 depending on conditions.
Primary halides, however, almost universally favor SN2, constrained by strong steric hindrance that blocks backside nucleophilic attack. “Primary substrates rarely form stable carbocations,” explains Chen, “so SN2’s single binding event remains energetically advantageous.” Methyl halides, lacking any stabilizing alkyl groups, typically proceed via SN2 or SN1 under very specific conditions—rarely achieving pure substitution without rearrangement or elimination.
Bulkier substrates, increased steric hindrance, and solvent polarity collectively steer reactivity.
Polar protic solvents such as water and alcohols stabilize carbocations and facilitate the stepwise dissociation essential for SN1, while polar aprotic solvents like acetone or DMSO accelerate SN2 by minimizing nucleophile solvation and enhancing attack efficiency.
Kinetics and Rate Dependence: Unimolecular vs. Bimolecular Reactions
The kinetic profiles of SN1 and SN2 further define their practical applications. SN1 reactions follow a first-order rate law: rate = *k*[substrate], because only the substrate participates in the slow, rate-determining step.“This simplicity allows precise kinetic modeling,” says Dr. Chen. “But it also means SN1 rates are less sensitive to nucleophile concentration—those molecules matter most in the beginning.” SN2, conversely, operates under second-order kinetics: rate = *k*[substrate][nucleophile].
“Here, both reactants influence the rate, making concentration control vital,” Smith clarifies. “A stronger nucleophile not only speeds up the reaction but may shift equilibrium or side reactions—something SN1 avoids by isolating leaving group departure.” This kinetic contrast explains why SN1 dominates in protic, high-temperature conditions, whereas SN2 thrives in aprotic, low-polarity environments.
Field studies in pharmaceutical synthesis reveal how this kinetic divide translates: SN1’s efficiency with weak nucleophiles enables streamlined, high-yield fertilizer for large-scale manufacturing, while SN2’s specificity supports asymmetric synthesis, where stereocontrol is nonnegotiable.
Stereochemical Outcomes: Inversion, Racemization, and Retention
One of the most observable differences lies in stereochemistry.SN1 reactions routinely produce racemic mixtures due to carbocation flexibility—nucleophiles attack from both sides of the flat intermediate—leading to invariant racemization. “A chiral center in an SN1 reaction loses its orientation,” explains Markov. “This limits its utility if stereochemical integrity is required.” SN2, by contrast, delivers clean inversion: the incoming nucleophile displaces the leaving group with predictable stereochemical reversal.
“This inversion makes SN2 indispensable in synthesizing enantiomerically pure compounds,” notes Dr. Markov. “From chiral pharmaceuticals to natural products, inverting configuration is often the difference between efficacy and inactivity.”
This stereochemical duality shapes application domains: SN1 suits racemic mixtures or flexible intermediates, while SN2 dominates in stereochemically precise syntheses, where each reaction type offers unique control.
Comparative Challenges and Practical Limitations
Both mechanisms present distinct industrial and synthetic challenges.SN1’s reliance on carbocation stability limits its use with primary substrates and complicates regioselectivity—multiple carbocation rearrangements can occur, yielding unintended byproducts. “Control of once-departing leaving groups demands precision,” warns Smith. “A single secondary halide may form both kinetic and thermodynamic products if conditions aren’t tightly managed.” SN2, though stereochemically precise, demands strong nucleophiles and environmentally consonant conditions.
“Bulky nucleophiles or unwieldy substrates often inhibit SN2,” Smith adds. “And steric congestion can derail the concerted mechanism entirely, forcing side reactions like elimination instead.”
Balancing these trade-offs defines synthetic strategy: SN1 excels when robust intermediates build yield, while SN2 delivers control when configuration is paramount. Mastery of both empowers synthetic chemists to navigate complex reaction landscapes with confidence.
The choice between SN1 and SN2 is not merely academic—it is a strategic decision shaping reaction efficiency, product selectivity, and industrial viability.By understanding mechanistic nuances, substrate behavior, and kinetic profiles, chemists transform abstract theory into precise practice. In the rh菌 of substitution chemistry, SN1 and SN2 stand as complementary pillars—each indispensable, each powerful in its domain. Their comparison underscores a core truth in organic synthesis: mastery of reaction mechanisms elevates every molecular transformation.


Related Post

Jiffy Lube Transmission Fluid Change Cost Revealed—What Ny Stewart Exposed in Episode 57 Shocked the Industry

Ingles Grocery Mills: The Heart of Nord’s Fresh Food Supply on the River — A Local Bákery, Mills, and Community Hub

When Is Kat Timpf Due? The Shocking Truth Behind Her Uncertain Delivery Amid Personal Tragedy and Political Tensions

The Ultimate Guide to the Top 50 Rappers of the Moment