The Nitrogen Atom Decoded: Unveiling Its Lewis Structure and Its Pivotal Role in Chemistry
The Nitrogen Atom Decoded: Unveiling Its Lewis Structure and Its Pivotal Role in Chemistry
Nitrogen, a lightweight yet indispensable element in the universe, lies at the heart of life, industry, and chemical innovation. As a fundamental building block of amino acids, nucleic acids, and the Earth’s atmosphere, nitrogen’s unique electronic behavior stems directly from its Lewis structure—a visual representation that reveals the atom’s bonding capacity and reactivity. Understanding nitrogen’s Lewis structure is not only essential for grasping its chemical behavior but also foundational for exploring biological processes, industrial applications, and environmental dynamics.
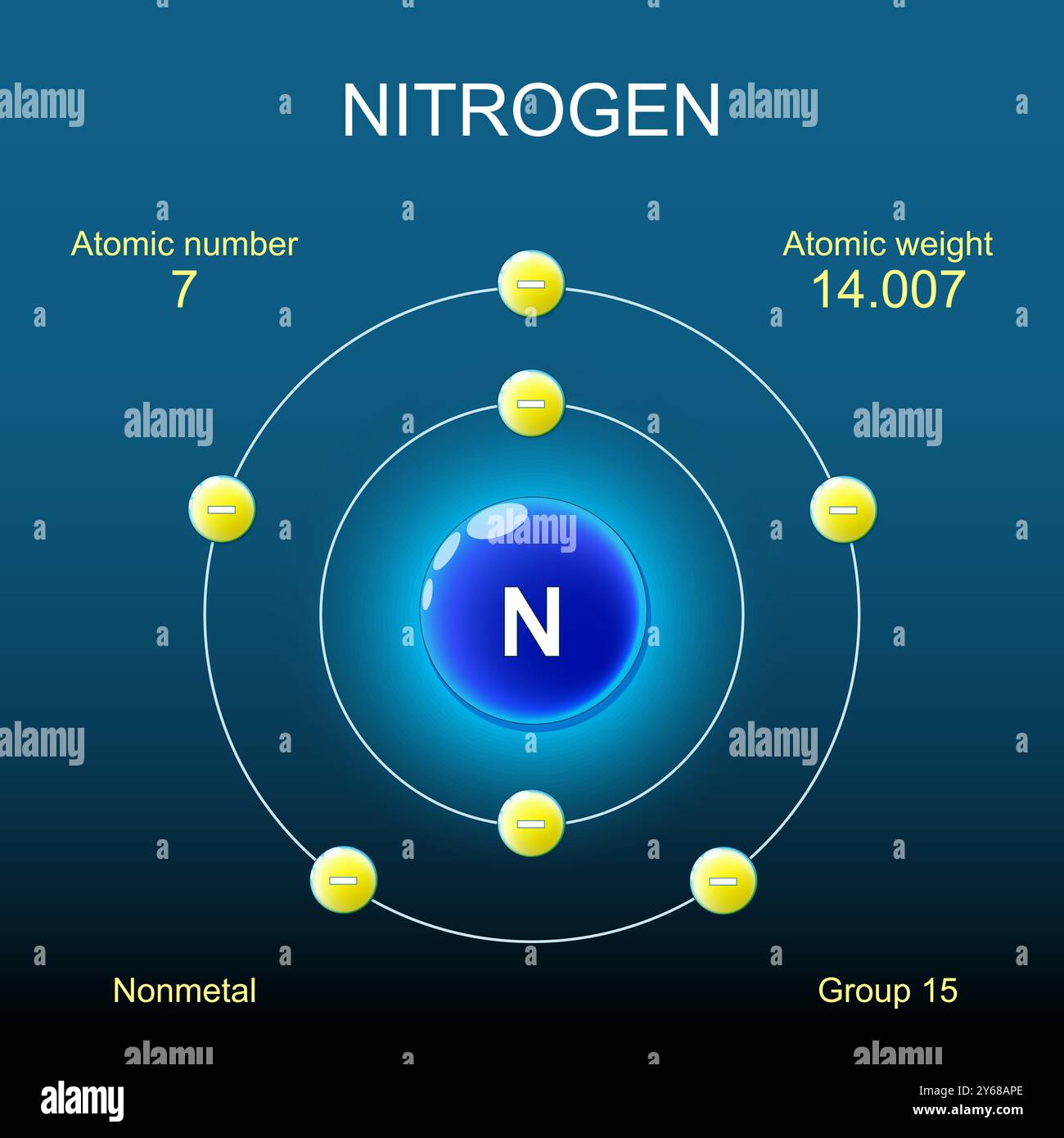
At the core of nitrogen’s chemical identity is a simple atomic configuration: with an atomic number of 7, the atom possesses seven electrons distributed across two electron shells. Its ground-state electron arrangement is 1s² 2s² 2p³, placing three electrons in the second subsidiary shell (2p orbital). This configuration is central to nitrogen’s remarkable ability to form diverse covalent bonds, particularly in molecules like ammonia (NH₃) and nitrogen gas (N₂)..
The Lewis Structure of Nitrogen: A Snapshot of Valence Electrons
Nitrogen’s valence electron count—five, derived from the two 2s electrons and three 2p electrons—determines its bonding patterns.Despite having eight electrons total in a stable formal octet, nitrogen typically forms three bonds to satisfy the octet rule, with one unpaired electron remaining in its 2p orbital. This odd number explains nitrogen’s paramagnetic nature and reactivity in free radical chemistry.
Drawing the Lewis structure reveals nitrogen centered with three single bonds to hydrogen atoms: N≡H—H (though commonly written as N–H–H). Each hydrogen contributes one electron, forming stable covalent single bonds, with nitrogen completing its outer shell through bonding rather than gaining or losing electrons.
This minimal, efficient arrangement underscores nitrogen’s role as a versatile participant in organic and inorganic chemistry. Unlike period 2 elements, nitrogen’s small size and high electronegativity (3.04) favor sharing electrons in polar covalent bonds, influencing its behavior in biological systems and synthetic processes alike.
The Role of Parity: Nitrogen’s Unique Electron Configuration
Nitrogen’s electron configuration sets it apart in the periodic table. As the only period 2 element with unsaturated p orbitals, it can achieve octet stability not only through full shells but also via efficient use of available p orbitals.The 2p³ arrangement allows nitrogen to form three high-quality covalent bonds, with one unpaired electron—critical for its participation in redox reactions and radical mechanisms. This paradoxical mix of stable octet readiness and transient electron lone-pair reactivity makes nitrogen both a responsive and predictable partner in chemical interactions, enabling everything from atmospheric N₂’s stability to the dynamic activity of ammonia in biological catalysis.
Despite its simplicity, nitrogen’s Lewis structure unlocks profound insights: from its inertness as diatomic N₂—linked to the strength of its triple bond (N≡N)—to its reactive nature in ammonia’s nucleophilicity.
Understanding this structure bridges fundamental theory and real-world impact, guiding advances in medicine, agriculture, and sustainable chemistry.
Nitrogen’s Geographic and Environmental Footprint
Nitrogen’s ubiquity belies its complex environmental journey. Although atmospheric N₂ constitutes 78% of dry air, its triple bond renders it largely inert, requiring energy-intensive processes—like the Haber-Bosch synthesis—to convert it into bioavailable forms for fertilizers. This transformation, while critical for global food production, underscores nitrogen’s double-edged role: essential yet potentially disruptive when mismanaged.Excess nitrogen runoff drives eutrophication in waterways, fueling harmful algal blooms and dead zones, while industrial emissions contribute to acid rain and greenhouse gas cycles. Ice core records reveal that human activity has doubled the global nitrogen cycle since the 20th century, altering natural balances with lasting ecological and climatic consequences.
In cellular contexts, nitrogen’s Lewis structure illuminates its biological importance.
In amino acids and nucleotides—building blocks of proteins and DNA—the precise tetrahedral geometry derived from nitrogen’s sp³ hybridization (though lone-pair repulsion limits full idealization) enables precise molecular docking and enzyme-substrate interactions. Nitrogen’s ability to stabilize charges through electron donation or acceptance underpins key biochemical pathways, including nitrogen fixation by bacteria and the urea cycle in mammals.
The Future of Nitrogen Science
As global demand for sustainable nitrogen management grows, deepening understanding of nitrogen’s fundamental structure drives innovation. Researchers optimize nitrogen-fixing enzymes, develop greener synthesis methods, and model nitrogen cycling in complex ecosystems with unprecedented precision.The Lewis structure, though elementary, remains a gateway to these frontiers—linking atomic behavior to planetary-scale challenges. From carbon-neutral ammonia production to mitigating nitrogen pollution, this simple representation continues to guide breakthroughs that shape chemistry, ecology, and human survival.
Nitrogen’s story, told through its electron cloud and bonding capacity, is far from trivial.
It is a narrative woven into life’s machinery, industrial infrastructure, and environmental systems—a testament to how the smallest atoms can steer the largest transformations. Mastery of nitrogen’s structure is not just a matter of academic curiosity; it is a cornerstone of responsible science in a changing world.


Related Post

The Electron Puzzle of Nitrogen Oxide: A Deep Dive into Its Lewis Structure and Reactive Nature

OSCOILS: Top Drilling Company In Oman

Karishma Kapoor A Look Back At Her Youthful Photos: Timeless Beauty Captured in Key Eras
Unlock Every Challenge Beneath Hyrule: Mastering the Legend of Zelda: Breath of the Wild Shrine Map

