Unlocking Biological Precision: The Role of Ion Charge in Calcium Function
Unlocking Biological Precision: The Role of Ion Charge in Calcium Function
Calcium ions (Ca²⁺) stand at the center of cellular communication, muscle contraction, skeletal integrity, and neural signaling—they conduct vital electrochemical messages that sustain life. Yet, the subtlety of their impact hinges on a fundamental physical property: ion charge. Specifically, the +2 charge of calcium ions governs their interactions, transport, and reactivity within biological systems.
Understanding how ion charge shapes calcium’s behavior reveals not just a cornerstone of biochemistry, but a key to breakthroughs in medicine and biotechnology. Calcium’s ionic charge of +2 is not a trivial detail—it is the driving force behind its biological significance. This divalent charge enables calcium to interact strongly with negatively charged molecules, most notably phosphate groups in ATP and DNA, as well as with cell membrane receptors and signaling proteins.
The high charge density enhances calcium’s ability to bind targets with precision, triggering cascades essential for cell function. "Why is +2 so decisive?" observes Dr. Elena Marquez, a biophysicist at the Institute for Cellular Energy Studies.
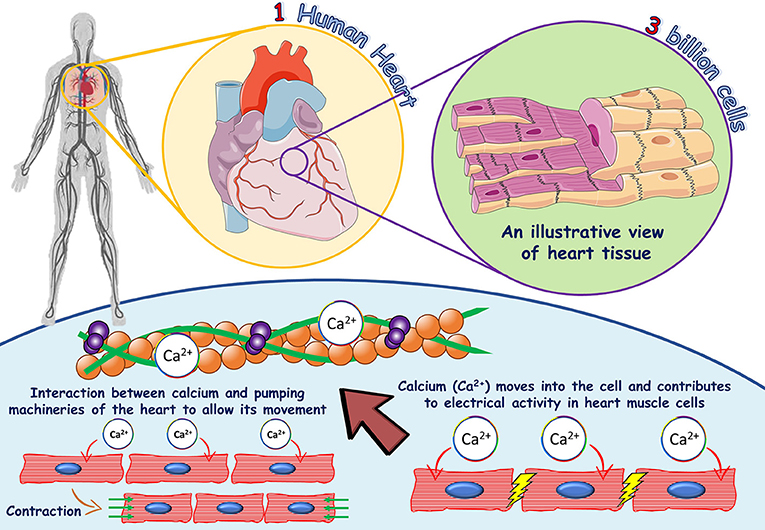
"Unlike monovalent ions such as sodium or potassium, calcium’s double charge allows for far more specific and energetically favorable interactions. This selectivity is critical in processes like muscle contraction, synaptic transmission, and bone mineralization—where timing and spatial control are non-negotiable." Ion charge dictates calcium’s movement across membranes and within cells. Due to its strong electrodal forces, Ca²⁺ channels open and close with exquisite sensitivity to membrane potential, enabling rapid signaling.
Calcium’s charge also promotes condensation around binding sites, reducing the energy barrier for wave-like propagation of calcium ions in neurons and muscle fibers. Without this charge, the very architecture of cellular communication would collapse. How Ion Charge Governs Calcium Transport and Signaling Calcium channels—protein pores embedded in cell membranes—rely entirely on electrostatic principles governed by ion charge.
These channels discriminate with precision between mobile ions based on charge and size, and calcium’s +2 charge is a master key to selective access. Voltage-gated calcium channels, for example, open only when membrane voltage shifts sufficiently, allowing Ca²⁺ influx predominantly through channels tuned to its unique charge. Ligand-gated channels, activated by neurotransmitters or intracellular messengers, bind calcium ions through negatively charged amino acid residues—interactions that thrive because of the ion’s double positive charge.
These molecular gatekeepers ensure that calcium enters cells precisely where and when needed: in synaptic terminals to prompt neurotransmitter release, in cardiomyocytes to trigger heartbeat contraction, or in osteoblasts to mineralize bone matrix. As Dr. Rajiv Patel, a molecular biologist at the National Institutes of Health explains, “Every calcium signal is a choreographed event—ion charge coordinates the opening, closing, and timing of channels, ensuring biological fidelity.” Beyond transport, calcium’s charge powers intracellular signaling cascades.
When Ca²⁺ enters a cell, it binds calmodulin and other calcium-binding proteins, changing their conformation and triggering downstream enzymes. This ion-driven cascade controls gene expression, metabolic activity, and cell survival. The electrical intimacy of charge reveals calcium as more than a mineral: it is a dynamic messenger.
The understanding of calcium’s ion charge opens vast frontiers in medicine and biochemistry. Therapies targeting calcium signaling are increasingly focused on modulating channel selectivity, with drugs like calcium channel blockers treating hypertension, arrhythmias, and stroke by fine-tuning ion flow. These pharmaceuticals exploit the ion’s charge to achieve specificity—blocking overactive channels without disrupting normal signaling.
In regenerative medicine, calcium’s role in bone mineralization highlights how ion charge supports structural integrity. Osteoblasts deposit calcium bound to phosphate in hydroxyapatite crystals only when ion charge enables proper binding. Disrupted charge—due to deficiencies in magnesium or vitamin D—compromises this process, linking calcium’s electrostatic nature directly to skeletal health.
Calcium’s charge also influences design in biomedical engineering. Researchers developing biosensors and artificial tissues use nanomaterials functionalized with charged peptides to mimic calcium’s biological interactions. These innovations promise better diagnostics and more responsive implants.
"We are no longer just observing calcium—we are learning to speak its ionic language," says Dr. Marquez. "Its +2 charge is not just a property; it is the punctuation mark in the grammar of cellular life." The charge of calcium ions is far more than a chemical curiosity—it is the silent conductor of life’s most vital processes.
From neuromuscular transmission to bone formation, this single electrostatic charge enables precision, energy efficiency, and selectivity that no other ion can match. As research deepens, harnessing ion charge in calcium’s behavior promises transformative advances across medicine and biotechnology, unlocking new treatments and benchmarks in human health.


Related Post
Is Hampton Inn A Marriott Hotel? Uncovering the Brand Connection and True Identity

Crawlist: The Structured Web Crawling Engine Redefining Data Collection in the Information Age

Jackie Defusco Married: Inside the Personal Life of a Prominent Figure

From Appalachia to the Screen: How the Hillbilly Elegy Movie Cast Ignited Cultural Conversation

