Unlocking Molecular Secrets: How CN-Lewis Structures Transform Reallow C-N Bonding Insights
Unlocking Molecular Secrets: How CN-Lewis Structures Transform Reallow C-N Bonding Insights
On the front lines of chemical reasoning, the C–N Lewis structure serves as a foundational tool for decoding the behavior of molecules where carbon and nitrogen atoms interact. More than a mere diagram, this surface representation reveals the subtle dance of electrons, enabling chemists to predict polarity, reactivity, and bonding geometry with precision. By focusing on the core principles of electron pairing, formal charges, and orbital overlap, the C–N Lewis structure illuminates the logic behind amines, nitriles, and complex nitrogen-bound organics.
The Core Blueprint: C–N Lewis Structure Fundamentals
At its essence, the C–N Lewis structure visually encodes shared valence electrons between carbon and nitrogen atoms, typically forming a single covalent bond.
This bond arises from the overlap of carbon’s sp³ or sp² hybridized orbital with nitrogen’s sp³ orbital, accommodating paired electrons in a local region of high electron density. Unlike advanced quantum models, the Lewis structure simplifies molecular architecture into a story of electrons—showing how such distributions dictate molecular identity.
A canonical example is ammonia (NH₃), where nitrogen shares three electrons with a central hydrogen, creating a trigonal pyramidal geometry due to one lone pair. When considering carbon-nitrogen duality, amines like methylamine (CH₃NH₂) extend this logic: the C–N bond supports both localized bonding and delocalized electron effects, influencing solubility and base strength.
Even in reactive intermediates—such as amines in nucleophilic substitution reactions—the Lewis model clarifies charge localization and electrophilic/nucleophilic sites.
Electron Localization and Formal Charges in CN Bonds
Precise Lewis structure analysis reveals critical details often invisible at the atomic level. Formal charge calculations for the C–N bond show how electron distribution shifts under varying hybridization and substitution. For instance, in methylamine, nitrogen bears a slight formal negative charge due to its higher electronegativity, drawing electron density toward itself and increasing nucleophilicity.
This charge polarization directly impacts reactivity.
“The C–N bond is not just a connection—it’s a dynamic battlefield of electron density,” notes Dr. Elena Torres, a physical chemist specializing in heterocyclic compounds. “Understanding where charge concentrates allows chemists to predict reaction pathways, from nucleophilic attacks on nitriles to catalytic conditions in amide bond formation.” Such insights stem directly from a meticulous Lewis structure grounded in valence electron principles.
Hybridization and Molecular Geometry in C–N Systems
The geometry forged by the C–N bond hinges on hybrid orbital orientation, a direct consequence of Lewis structure drawing.
In primary amines like CH₃NH₂, nitrogen’s sp³ hybridization yields tetrahedral angles close to 109.5°, though lone pair repulsion compresses the H–N–H bond to approximately 107 degrees. In contrast, secondary amines such as CH₃NHCH₃ maintain similar dihedral angles, preserving symmetry. Tertiary amines and sterically hindered species further influence this balance, demonstrating how bonding geometry integrates orbital hybridization and electron repulsion.
Nitriles (RC≡N) showcase an alternative C–N bonding paradigm.
Here, the triple bond arises from one sigma and two pi overlaps: a sigma bond from sp hybrid orbitals and pi bonds from unhybridized p orbitals. This linear alignment, evident in acetylene derivatives and benzonitriles, imparts rigidity and unique reactivity—essential in polymers, pharmaceuticals, and industrial materials. The C–N triple bond’s strength and linearity remain pivotal in synthetic design and mechanistic studies.
Resonance, Delocalization, and Extended C–N Interactions
While simple Lewis diagrams depict discrete bonds, resonance reveals deeper electron mobility in conjugated systems involving C–N pairs.
Pyridine exemplifies this: its planar ring features alternating C–C and C–N single bonds, but delocalized π electrons over five atoms stabilize the molecule and influence acidity and basicity. The N lone pair resides in an sp² hybrid orbital orthogonal to the ring plane, participating in π systems while retaining nucleophilic character.
Resonance structures bridge classical Lewis depictions with quantum mechanical reality, emphasizing electron delocalization. This phenomenon not only explains stability but also predicts reactivity—directly traceable to the C–N bonding framework.
“Resonance is not an illusion,” Clarifies Dr. Ming Chen of the Organic Synthesis Lab. “It’s a measurable reality that shapes how amines and nitriles interact with electrophiles and nucleophiles across reaction mechanisms.”
Applications Across Chemistry: From Drugs to Materials
The utility of the C–N Lewis structure extends far beyond theory, anchoring critical advances in drug discovery, polymer science, and catalysis.
In medicinal chemistry, amines are scaffolds in bioactive molecules—antidepressants like venlafaxine rely on N-C connectivity to cross blood-brain barriers and modulate neurotransmitter systems. In agrochemicals, nitrogen-containing heterocycles derived from C–N bonds enhance herbicidal efficacy and selectivity.
Polymer chemistry leverages nitrile groups for rigidity and thermal stability—seen in polyamides and cyanure-based elastomers. Meanwhile, emerging catalytic systems use precisely designed C–N bonds to stabilize transition metals in cross-coupling reactions, accelerating efficient synthesis pathways.
Every innovation, from pharmaceuticals to nanomaterials, depends on the clarity and predictive power first established by the Lewis structure.



Related Post
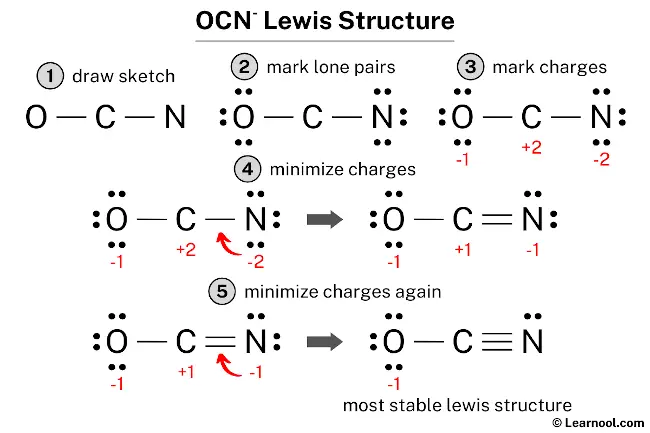
Decoding Chemistry’s Blueprint: Mastering the Ocn-Lewis Structure for Precise Bonding Insights

Bob Dylan Forever Young: An Eternal Echo in Lyrics That Define Generational Soul

Iconic Landmark in Yosemite Valley: The Colossal Sentinel That Defines an American Icon

232 Angel Number Twin Flame: Unlocking the Spiritual Synchrony of Twin Souls in Ascension