Unlocking the Chemistry Behind Hydrogen Cyanide: The Precision of Its Lewis Structure
Unlocking the Chemistry Behind Hydrogen Cyanide: The Precision of Its Lewis Structure
The Molecular Blueprint of a Key Industrial and Synthetic Building Block
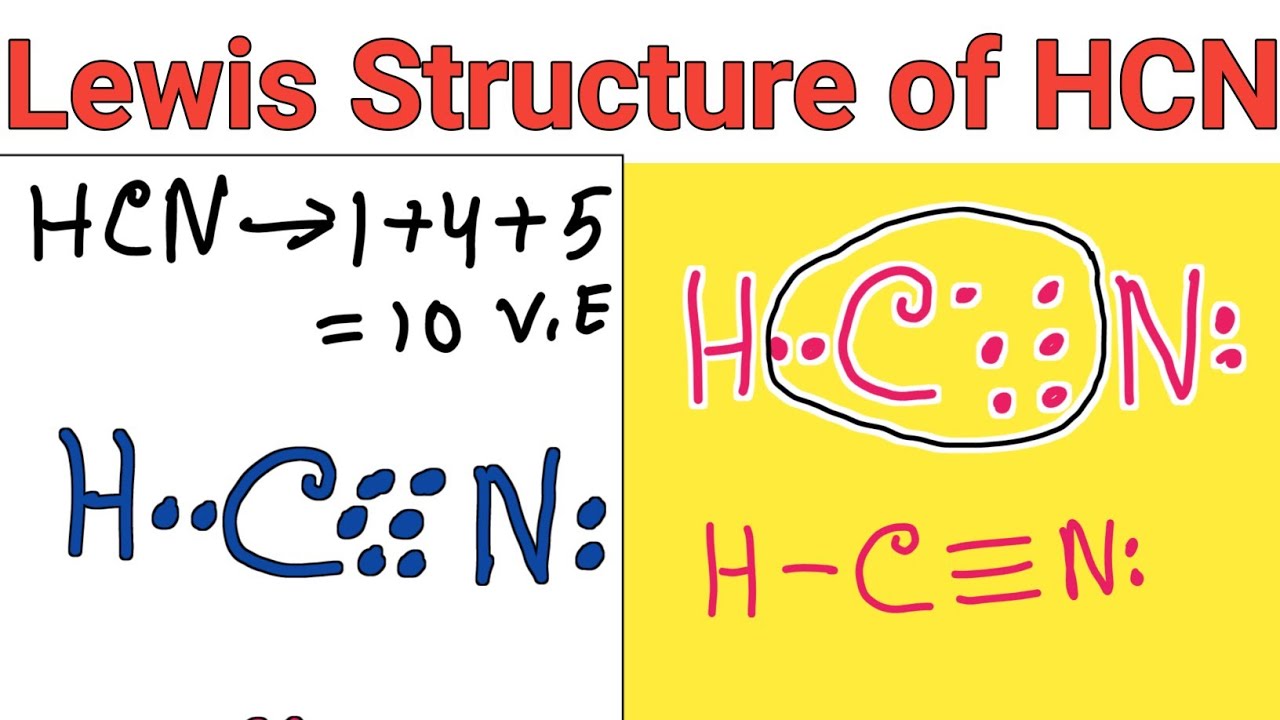
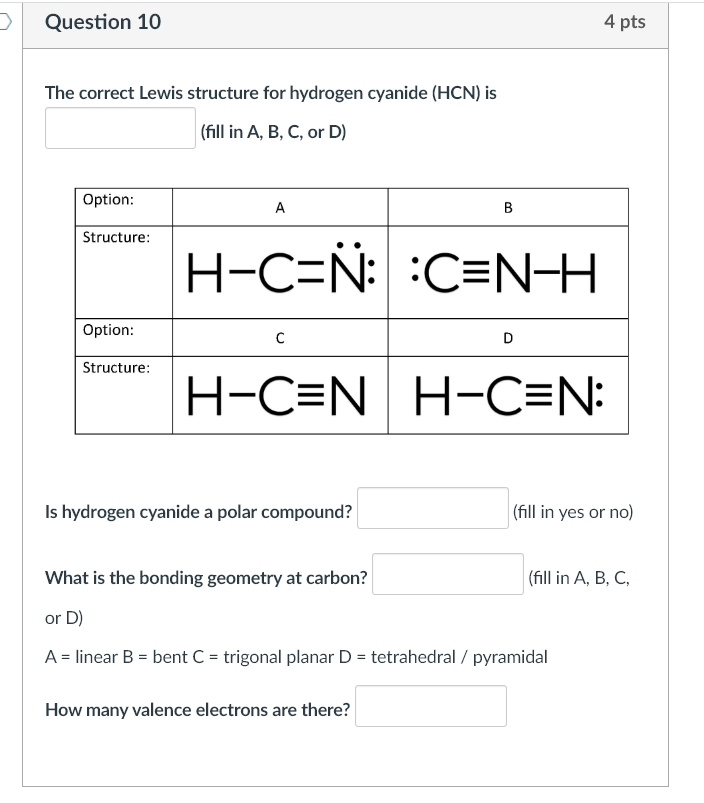
Hydrogen cyanide, a compound of profound industrial importance, serves as a critical precursor in the synthesis of pharmaceuticals, agrochemicals, and polymers. Its molecular structure, elegantly defined by the Lewis structure, reveals the precise arrangement of electrons and atoms that underpin its reactivity and utility. Understanding the Lewis structure of hydrogen cyanide—formally represented as H–C≡N—provides essential insight into its chemical behavior and wide-ranging applications.This article explores the atomic layout, bond characteristics, and functional implications encoded in its structure, emphasizing why this simple yet powerful molecule is indispensable across science and industry.
At the core of hydrogen cyanide lies a terminal carbon atom triple-bonded to a nitrogen atom, with a hydrogen atom covalently attached—forming H–C≡N. This arrangement follows the fundamental tenets of valence shell electron pair repulsion (VSEPR) theory, where electron domains dictate molecular geometry.
The triple bond consists of one sigma (σ) bond and two perpendicular pi (π) bonds, contributing to both bond strength and stability. The linear configuration—H–C≡N—minimizes electron repulsion, yielding a molecule with consistent bond angles near 180 degrees, a hallmark of sp-hybridized carbon and carbon-nitrogen bonding.
The Role of Electron Distribution and Hybridization
The carbon atom in hydrogen cyanide undergoes sp-hybridization, mixing one 2s electron and three 2p orbitals to form four equivalent hybrid orbitals. Three of these orbitals form σ bonds—with hydrogen and the two side atoms—while the remaining p orbital engages in pi conjugation across the C≡N bond.This hybridization not only defines the linear geometry but also affects the electron density distribution: nitrogen’s lone pair resides in an unhybridized p orbital, contributing to the molecule’s polarity and basic characteristics. Unlike terminal hydrides, which favor saturated bonding, hydrogen cyanide’s unsaturated triple bond grants unique reactivity, enabling nucleophilic additions and resonance stabilization in various reaction environments.
Electron Count and Lewis Symbolism
A detailed Lewis structure representation offers clarity on electron occupancy and bonding economy. The core structure features five total valence electrons from hydrogen, seven from carbon, and five from nitrogen—totaling 17 valence electrons, consistent with standard covalent bonding patterns.In H–C≡N, four electrons form the triple bond (H−C≡N), while one electron remains as a lone pair on nitrogen, consistent with its +3 oxidation state and inert pair characteristics. Representing excess electrons and formal charges reveals no significant formal charge imbalance, underscoring the molecule’s chemical neutrality under standard conditions. This electron balance enhances stability, reducing susceptibility to uncontrolled decomposition—a vital trait in storage and processing.
Despite its simplicity, hydrogen cyanide’s structure exemplifies how subtle electronic features drive profound chemical behavior. Its triple bond imparts durability against hydrolysis and oxidation, while the polar N–C bond enables selective chemical transformations—key factors behind its application as a versatile synthetic intermediate. In pharmaceutical synthesis, for example, the cyanide group can be transformed into carboxylic acids, amides, or amines, illustrating the molecule’s dynamic reactivity.
Moreover, its dipolar nature facilitates solubility and interaction with catalysts, accelerating complex organic reactions essential in drug development.
Industrial and Environmental Implications
Beyond synthetic utility, understanding the Lewis structure deepens awareness of hydrogen cyanide’s environmental footprint. As a gas with strong odor detectable at parts-per-million levels, its reactive nature demands careful handling. Industrial processes leveraging its structure prioritize closed systems and efficient catalytic conversions to minimize toxic release.Research into green chemistry approaches seeks to harness its reactivity while reducing hazard—mirroring efforts seen in handling other nitrogen-based synthons. The molecule’s design thus balances functionality with safety, informing modern standards in chemical manufacturing and environmental stewardship.
The meticulous arrangement of atoms in hydrogen cyanide, captured through its Lewis structure, reveals not just a static image but a dynamic framework governing reactivity, stability, and application.
Its triple bond and liganded hydrogen define a template of electron distribution and hybridization that chemists exploit to design targeted transformations. Far from a mere industrial reagent, hydrogen cyanide stands as a textbook example of how structural precision enables functional utility across diverse sectors. Mastery of its Lewis structure empowers forward-thinking innovation in synthesis, safety, and sustainability—affirming its status as a cornerstone of modern chemistry.


Related Post

Sugary Spire: The Most Irresistibly Sweet Showcase in Dessert’s Global Empire

Truly Master LTRS Unit 6 Session 2 Check For Understanding: The Power Behind Letter Reconnection

Kwite Face: Revolutionizing Digital Communication Through Real-Time Multimodal Emotion Recognition

Who Is Lisa Boothe’s Husband? Meet John Bourbonia Cummins III

