Unlocking the Geometry of Reactivity: A Deep Dive into the Pcl5 Lewis Structure
Unlocking the Geometry of Reactivity: A Deep Dive into the Pcl5 Lewis Structure
In the intricate world of molecular chemistry, understanding how atoms arrange themselves around a central element determines a compound’s behavior, stability, and reactivity. Nowhere is this clearer than in the pentachlorophosphorus molecule, Pcl₅, whose electron-pair distribution, as defined by its Lewis structure, reveals fundamental principles of inorganic chemistry. The molecule’s unique structure—featuring five chlorine atoms clinging to a central phosphorus atom—serves as a textbook example of hypervalency, offering key insights into coordination chemistry and molecular polarity.
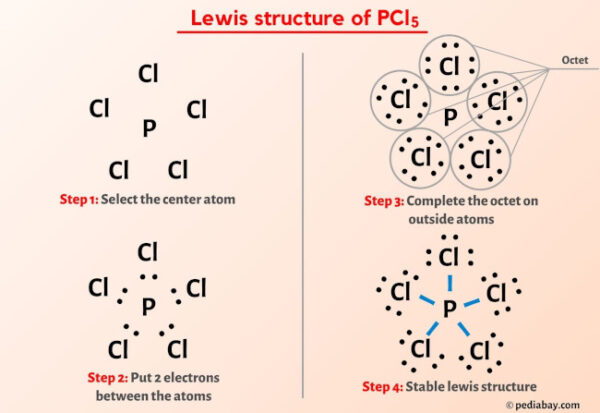
At the core of Pcl₅’s chemistry lies its Lewis structure: a phosphorus atom at the center with five single bonds to chlorine atoms and no lone pairs.
This configuration departs from the octet rule, illustrating the phenomenon of hypervalency, where central atoms accommodate more than eight valence electrons. “Pcl₅ exemplifies how electron-deficient phosphorus expands its coordination sphere,” notes Dr. Elena Rodriguez, a molecular chemist at Stanford University.
“The absence of lone pairs and the presence of five bonding regions give the molecule a trigonal bipyramidal geometry—key to its ligand behavior and reactivity.”
Key Atomic Arrangement: From Octet to Hypervalency Contrary to the traditional octet rule, phosphorus in Pcl₅ exhibits a valence of five formal bonds. With no lone electron pairs and five bonding electron domains, the molecule assumes a trigonal bipyramidal geometry. This arrangement consists of three equatorial positions forming a triangular plane and two axial positions perpendicular to this plane.
Each phosphorus-clorine bond is a single covalent linkage, with electrons distributed symmetrically to minimize repulsion, as predicted by VSEPR (Valence Shell Electron Pair Repulsion) theory. The bond angles reflect this structure: 90° between axial and equatorial bonds, and 120° among the equatorial chlorine atoms. This arrangement maximizes spatial separation, stabilizing the molecule despite its five appended ligands.
Electron Distribution and Molecular Polarity Despite multiple polar P–Cl bonds, Pcl₅ is a nonpolar molecule overall.
Each P–Cl bond exhibits significant dipole character due to chlorine’s high electronegativity (3.16 on the Pauling scale), pulling electron density toward Cl. However, symmetry eliminates net polarity: the trigonal bipyramidal shape ensures that bond dipoles cancel vectorially. “The symmetry of the molecular structure is a defining feature of Pcl₅’s stability,” explains Dr.
Mark Chen, a specialist in organophosphorus compounds. “This cancellation underpins its utility in catalysis, where consistent electron distribution avoids unwanted side reactions.”
Hypervalency and Bonding Theories Pcl₅ challenges traditional bonding models, requiring explanations rooted in molecular orbital theory and valence bond concepts. While standard VSEPR explains geometry, bonding demands more nuance.
The phosphorus atom employs sp³d hybridization, mixing one s, three p, and one d orbital to form five equivalent hybrid orbitals. Each orbital contains a shared electron pair with a chlorine atom, a standard model for hypervalent molecules. “The d-orbital involvement remains debated,” notes chemist Dr.
Sofia Moretti, “but the key takeaway is that phosphorus accesses additional coordination capacity through expanded valence shell behavior.” Quantum mechanical calculations reveal electron densities concentrated at bridgehead positions, reinforcing the molecule’s electron-rich central core.
Chemical Reactivity: Ligand Behavior and Redox Potential The vacant axial site in trigonal bipyramidal Pcl₅ makes it an excellent ligand in transition metal complexes. Because axial positions are slightly longer and lower in electron density, they preferentially bind to Lewis acids or undergo substitution under mild conditions. This property enables Pcl₅ to act as a precursor in synthesizing heterometallic compounds with tailored reactivity.
In redox chemistry, phosphorus exhibits +5 oxidation state, stable yet reactive. Substituents like Cl⁻ can be displaced via electron transfer, facilitating transformations in catalysis and organic synthesis.
Comparison with Structural Analogues Pcl₅ stands apart from simpler binuclear chlorides like Cl₂PCl₅ or POCl₃ due to its complete trigonal bipyramidal symmetry. While Pcl₅’s central phosphorus is bonded to five chlorines, analogs often include lone pairs or asymmetric substituents that perturb geometry and reactivity.
For instance, PBr₅—structurally similar—combines phosphorus with five bromines, resulting in a distorted tetrahedral geometry due to poorer radial distribution of larger bromine atoms. “The pristine symmetry of Pcl₅ provides predictable reactivity patterns ideal for mechanistic studies,” says Dr. Chen.
“It’s a rare compound that crystallizes so cleanly under standard conditions, offering a stable reference point for bond energetics.”
Applications in Industry


Related Post

Who Voices Knuckles? The definitive guide to the iconic Sonic antagonist’s voice pedigree

Future Trunks Saga Dragon Ball Z Explained

Chelsea Deboer Teen Mom 2 Bio Wiki Age: The Rise of a Young Mother Made Famous

How Many Cups Are in a Pound of Powdered Sugar? The Definitive Guide

