Unlocking the Power of Ethanol: The Molecular Formula Behind a Global Staple
Unlocking the Power of Ethanol: The Molecular Formula Behind a Global Staple
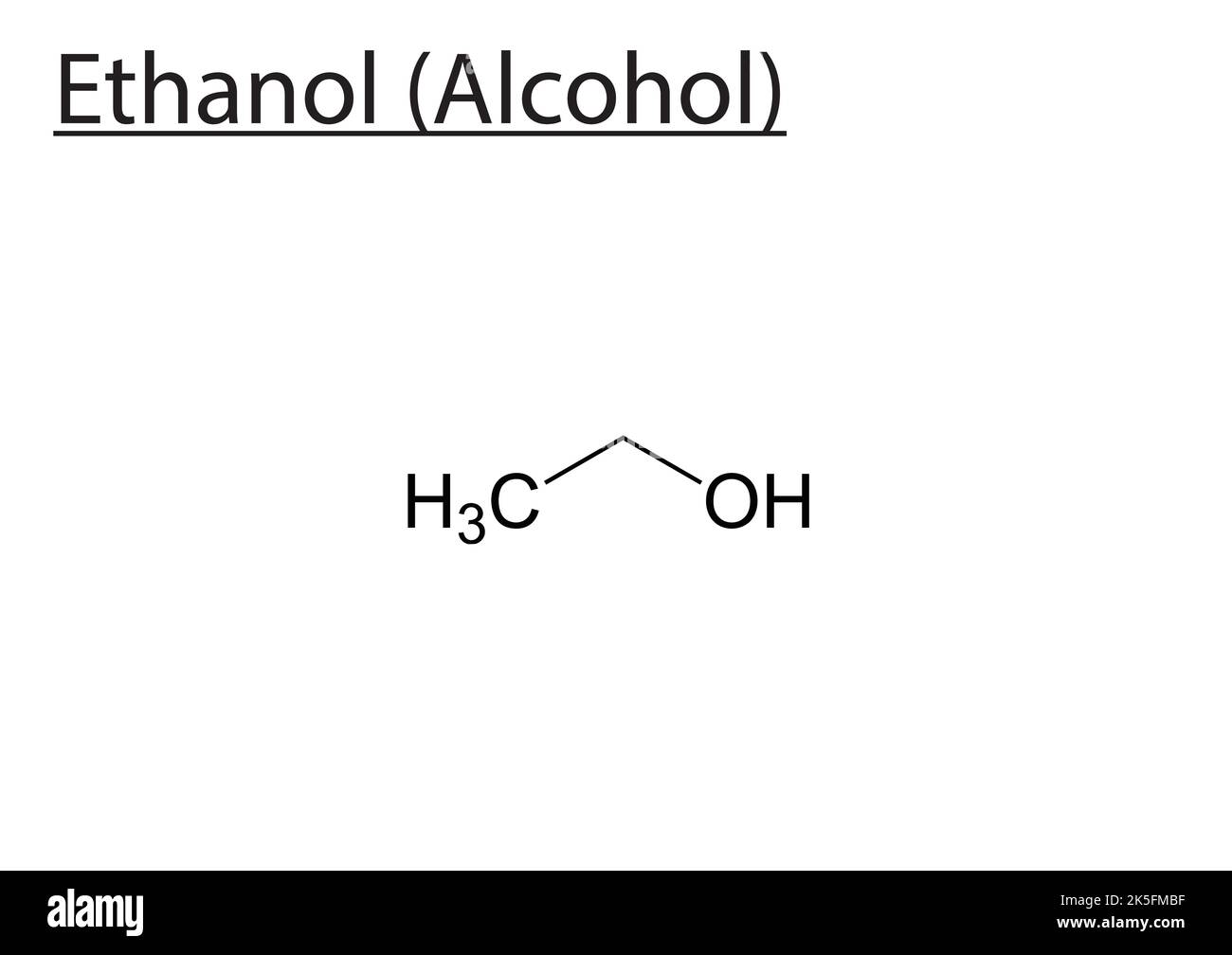
Molecularly, ethanol is defined by the formula C₂H₅OH—a simple yet transformative compound central to industries ranging from pharmaceuticals to beverages, and even biofuels. This small six-atom molecule, composed of two carbon atoms, six hydrogen atoms, and a hydroxyl-functionalized terminal oxygen, underpins one of the most widely consumed and studied organic compounds in science and everyday life. As both a solvent and a metabolic fuel, ethanol’s structure dictates its reactivity, solubility, and broad utility, making its molecular blueprint essential to understanding its role across multiple domains.
Molecular Configuration: The Blueprint of Ethanol – C₂H₅OH
Ethanol’s chemical identity is rooted in its molecular formula, C₂H₅OH. Breaking this down, two carbon atoms form the backbone, each bonded to a series of hydrogen and hydroxyl groups. The “C₂” reflects the two carbon units connected by a single or double bond depending on context—though in ethanol, both carbons carry single bonds in a linear chain.Attached to the second carbon is a hydroxyl group (–OH), a hallmark of alcohols, which imparts polarity and reactivity. The final “OH” attributes to ethanol’s hydrophilic character: the oxygen’s electronegativity creates a partial negative charge, enabling hydrogen bonding with water and polar molecules. “This combination of carbon, hydrogen, and a hydroxyl group makes ethanol amphiphilic—capable of interacting with both organic and aqueous environments,” explains Dr.
Elena Torres, organic chemist at the Institute for Biochemical Sciences. The formula C₂H₅OH also explains ethanol’s physical properties: a molecular weight of 46.07 g/mol, a boiling point of 78.37°C, and miscibility in water up to 100%, critical for its use in distillation and solution-based processes.
Composition Breakdown: Carbon, Hydrogen, and the Hydroxyl’s Functional Role
Ethanol’s molecular architecture is a model of functional simplicity.Carbon atoms, the structural foundation, account for 25% of the molecule (2 out of 8 total atoms), positioning each to form stable bonds with both hydrogen and oxygen. Hydrogen atoms, totaling 6, are distributed so that each carbon shares four hydrogen bonds, while the hydroxyl oxygen binds two hydrogens directly and forms one hydrogen bond with surrounding molecules. This distribution gives ethanol unique solvation properties: it readily dissolves in water by forming intermolecular hydrogen bonds, a trait exploited in laboratories, pharmaceuticals, and distilling.
The hydroxyl group also introduces reactivity uncommon in aliphatic alcohols—enabling oxidation to acetaldehyde and further to acetic acid, carbonation in beverages, and esterification with carboxylic acids. “The OH group is ethanol’s gateway to chemical transformation,” notes Prof. Michael Chen, a specialist in industrial organic chemistry.
“It not only defines solubility but unlocks pathways for synthesis, catalysis, and functional modification.”
Chemical Reactivity: From Oxidation to Solvation in Industrial and Biological Contexts
Ethanol’s reactivity hinges on its molecular formula and functional group interplay. The hydroxyl group allows redox transformations central to energy production and chemical manufacturing. In biological systems, ethanol is metabolized primarily in the liver via oxidation by alcohol dehydrogenase, converting it to acetaldehyde—a compound toxic in high concentrations—before progressing to acetate and ultimately CO₂ and water.This metabolic pathway underscores ethanol’s dual nature: a bioavailable energy source processed efficiently at low doses but dangerously cumulative at high intake. Industrially, ethanol’s reactivity enables ester formation—such as methyl ethyl ether production—used in solvents and plasticizers. Its rapid evaporation kinetics and moderate polarity make it ideal for disinfection, solvent formulations, and even as a biofuel additive, where blending with gasoline (via C₂H₅OH’s volatility and oxygen content) improves combustion efficiency.
“Ethanol’s versatility arises directly from C₂H₅OH’s balance of stability and reactivity,” states Dr. Sofia Mendez, expert in industrial chemistry at the Global Bioethanol Initiative.
Applications and Impacts: From Home Samples to Global Markets
The molecular characteristics of ethanol fuel a staggering array of applications.In consumer products, ethanol dominates alcoholic beverages—where its 40–50% ABV, derived directly from fermentation of C₂H₅OH, drives flavor, aroma delivery, and sensory experience. Pharmaceuticals rely on ethanol as a solvent and antimicrobial agent, leveraging its ability to denature proteins and disrupt microbial membranes. In healthcare, ethanol’s role in hand sanitizers—typically 60–70% C₂H₅OH—maximizes pathogen inactivation without excessive viscosity or residue.
The fuel sector exploits ethanol’s high octane rating and oxygen content, which enhance combustion cleanliness compared to pure hydrocarbons. “Ethanol’s molecular simplicity enables scalable, adaptable use,” observes Dr. Rajiv Patel, energy policy analyst.
“Its role in biofuels reduces fossil fuel dependence, while in medicine, its biocompatibility ensures broad application.”
Environmental and Safety Considerations: Balancing Use and Impact
While ethanol is often praised for its biodegradability and reduced carbon footprint—when derived from renewable feedstocks like corn or sugarcane—its large-scale production raises sustainability questions. Industrial fermentation, though renewable, demands significant water and energy inputs, prompting innovation in feedstock optimization and bioprocessing. Environmentally, ethanol’s low toxicity compared to methanol or formaldehyde supports safe handling, yet improper disposal or overuse in agriculture can strain ecosystems.“Ethanol’s story is one of utility shaped by chemistry,” says Dr. Torres. “Understanding its molecular formula C₂H₅OH reveals the dual edge of its widespread use: immense utility tempered by responsible management.”
In summary, ethanol’s molecular formula C₂H₅OH serves as more than a chemical label—it encapsulates a molecule of extraordinary functional breadth.
From its polar hydroxyl group enabling biological metabolism to its balanced carbon-hydrogen framework supporting industrial versatility, ethanol exemplifies how molecular design directly shapes global applications. As science advances, the compound’s story continues to evolve—grounded in its formula, yet extending into every drop of beverage, every lifeline in medicine, and every sustainable fuel advancement. Recognizing ethanol’s molecular truth deepens our appreciation of its role and informs how we harness it responsibly in a changing world.

/GettyImages-136810090-56a133b25f9b58b7d0bcfd93.jpg)

Related Post

Cool Kei Tenin San O Omochikaeri: The Soul of Japanese Punk Revival

Celta Vigo vs Getafe: A Battle of Stamina and Precision in La Liga’s Crucible — Preview, Predictions, and In-Depth Analysis
Unveiling The Enigmatic Journey Of Famke Janssen: From Swedish Stage to Global Screen Stardom

Lincoln Steffens Emerged as a Reform Champion Captured in Iconic “Photograph by Grenger Fine Rtc Meric”

