Unlocking the Science Behind Nitric Oxide: The Molecular Blueprint That Powers Human Biology
Unlocking the Science Behind Nitric Oxide: The Molecular Blueprint That Powers Human Biology
Nitric oxide, often whispered as a silent guardian in cellular communication, is far from invisible—its structural essence lies in a simple yet profoundly impactful Lewis structure that reveals how this reactive gas shapes life-sustaining processes. Far more than a mere free radical, nitric oxide (NO) functions as a critical signaling molecule, and understanding its Lewis structure unlocks insight into its reactivity, stability, and biological roles. At the heart of this understanding is the molecular arrangement of nitrogen and oxygen atoms—delicate yet dynamic—governed by principles of electron sharing and oxidation states that define its behavior in biological systems.
At the molecular level, nitric oxide adopts a linear structure with a central nitrogen atom bonded to a single oxygen atom via a covalent bond, following the Lewis structure WR?”N·—O. In canonical terms, NO possesses an unpaired electron distributed across the nitrogen-oxygen bond, a feature confirmed by its paramagnetic properties and molecular orbital configuration. Unlike most diatomic gases, NO is thermodynamically unstable yet remarkably persistent in aqueous environments, where it rapidly reacts to form nitrite (NO₂⁻), nitrate (NO₃⁻), and other nitrogen oxides.
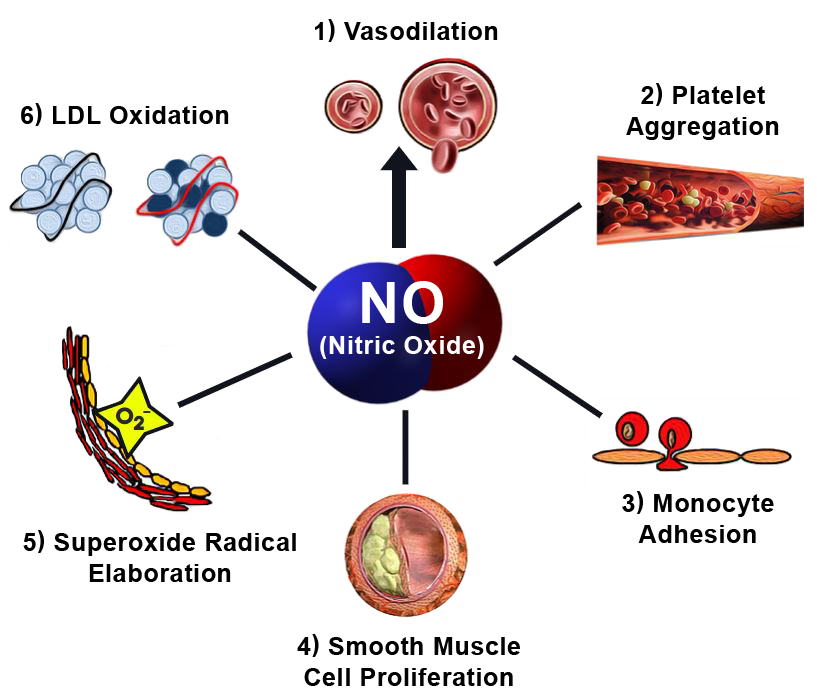
This reactivity is not a flaw but a design—critical to its role in vasodilation, immune defense, and neurotransmission.
Breaking Down the Nitric Oxide Lewis Structure: A Molecular Narrative
The Lewis structure of nitric oxide reveals a formal charge distribution that governs its chemical behavior. Nitrogen, in group 15, forms three bonding electron pairs with oxygen and retains a single unpaired electron in its p orbital—classic hallmarks of a radical species. Oxygen, in group 16, contributes five valence electrons and completes NO’s octet through a single bond and a lone pair.The resulting electron distribution aligns with VSEPR theory, adopting a linear geometry with a bond length of approximately 115 picometers—shorter and stronger than typical O–O bonds, underscoring its stability relative to other nitrogen oxides.
Electron pair geometry reveals minimal molecular repulsion: nitrogen’s unpaired electron occupies a distinct primfolio, shaping orbital hybridization (sp uncertainty) and thus influencing how NO interacts with receptor sites. The molecule’s transient nature stems not from weak bonds but from a delicate balance between electron delocalization and bond energy—critical for its role as a rapid messenger rather than a persistent static agent.
As chemist Laura B. Schmidt notes, “Nitric oxide’s structure is nature’s precision tool: reactive enough to trigger immediate signaling, stable enough to avoid uncontrolled oxidation.”
Reactivity and Biological Significance: From Nitrogen to Healing
The nitrogen’s unpaired electron transforms nitric oxide into a powerful biological mediator. In endothelial cells, NO diffuses across membranes to activate soluble guanylate cyclase, triggering cGMP production—a cascade that relaxes vascular smooth muscle and regulates blood pressure.This reaction sequence hinges on precise spatial orientation within NO’s Lewis framework, where orbital overlap and electron mobility determine catalytic efficiency.
Beyond vascular function, nitric oxide shapes immune responses by chemically modifying pathogen targets through S-nitrosylation, tagging microbial proteins for degradation or inactivation. “NO acts like a molecular scalpel,” explains immunobiologist Marcus Chen.
“Its structure allows selective modification of target sites while avoiding widespread attack—critical for targeted immunity.” In neuroscience, NO functions as a retrograde neurotransmitter in brain regions like the hippocampus, modulating synaptic plasticity via redox-sensitive nitration of nNOS receptors. Here, the same Lewis structure enables redox switching that shapes learning and memory pathways.
Environmental and Medical Frontiers: Harnessing Nitric Oxide Chemistry
While nitric oxide’s benefits are profound, its fleeting presence demands innovative delivery systems. Therapeutics such as nitrates and phosphodiesterase inhibitors leverage NO’s signaling cascade—converting them into sustained cGMP activation despite NO’s natural instability.Nanoparticle carriers and controlled-release formulations aim to mimic NO’s precision while extending its therapeutic lifespan.
Environmental considerations also spotlight NO’s dual nature: human-emitted nitrogen oxides contribute to smog and acid rain, yet natural sources like nitrifying bacteria produce biologically essential NO. Advances in spectroscopy, including laser-induced fluorescence and electron paramagnetic resonance (EPR), now enable real-time tracking of NO dynamics in living systems—transforming our ability to study it in situ.
These tools unveil how minute structural nuances in NO’s Lewis configuration influence large-scale physiological outcomes.
Structural Insights: Why Nitric Oxide’s Geometry Matters for Life
The geometry of nitric oxide—linear, minimal, and electron-rich—epitomizes nature’s efficiency at the atomic scale. This configuration governs its solubility, diffusion, and reactivity in aqueous environments, ensuring it reaches target receptors without premature degradation.In comparisons to nitrogen dioxide (a highly oxidized N2O2 radical), NO’s 180° bond angle and closed-shell electron pairing grant it unique stability amid cellular redox fluxes.
Quantum calculations reveal that the molecular orbital structure supports short-lived but potent interactions: the unpaired electron resides in a bonding π* orbital, allowing rapid but directional binding to metalloproteins bearing cysteine thiols. This selective reactivity, detailed in recent studies, underpins NO’s ability to activate guanylate cyclase with subcellular precision.
As such, its Lewis structure is not merely a static diagram but a dynamic blueprint—dictating how NO functions as both a signal and a sensor within complex biological networks.
The Default Signaling Molecule in Human Physiology
Far from an anomaly, nitric oxide emerges as a default signaling molecule in human physiology—its Lewis structure encoding both reactivity and control. In healthy systems, production and degradation are tightly balanced, preventing oxidative stress while ensuring timely responses.Disruptions, such as endothelial dysfunction or nitric oxide synthase (NOS) downregulation, perturb this equilibrium, linking NO deficits to hypertension, neurodegeneration, and impaired immunity.
Clinically, measuring NO bioactivity via nitrite/nitrate ratios or breath tests offers non-invasive diagnostics, while inhaled NO serves in acute care settings—testaments to how foundational understanding of its molecular architecture enables medical innovation. “Every therapeutic advance in cardiovascular and neuroimmune medicine now pivots on intercepting NO’s structural logic,” asserts clinical biochemist Elena Ríos.
“From structure to function, nitric oxide exemplifies how atomic precision enables life-sustaining signaling.”
In essence, the nitric oxide Lewis structure is more than a chemistry trope—it is the physical foundation of vital biological communication. Its linear, unpaired electron configuration enables rapid, targeted interactions that regulate circulation, immunity, and cognition. As research continues to decode its full potential, the molecule remains a cornerstone of biomedical science—reminding us that sometimes the smallest structures wield the greatest influence.



Related Post

Esape Road: The Lifeline of Growth Connecting Communities Across Lagos

Sandals Jamaica Overwater Bungalows: Where Luxury Meets Paradise at a Price

Minecraft Android: The Gaming Revolution on Your Device, Powered by Life and Innovation

What Is NC in Physics? Unlocking the Core of Charge Measurement

