Unlocking the Secrets of Cu Lewis Dot Structure: How Metal Electron Behavior Drives Chemical Reactivity
Unlocking the Secrets of Cu Lewis Dot Structure: How Metal Electron Behavior Drives Chemical Reactivity
Understanding the cu Lewis dot structure is fundamental to decoding how transition metals interact in chemical reactions, influencing fields from materials science to catalysis. By visually mapping valence electrons around copper atoms and their bonding partners, this simple yet powerful framework reveals the underlying electronic architecture that governs reactivity, oxidation states, and complex formation. Despite its apparent simplicity, the Cu Lewis dot structure highlights the nuanced quantum behavior of metallic elements, offering a bridge between abstract electron theory and real-world chemical phenomena.
What Is a Cu Lewis Dot Structure?
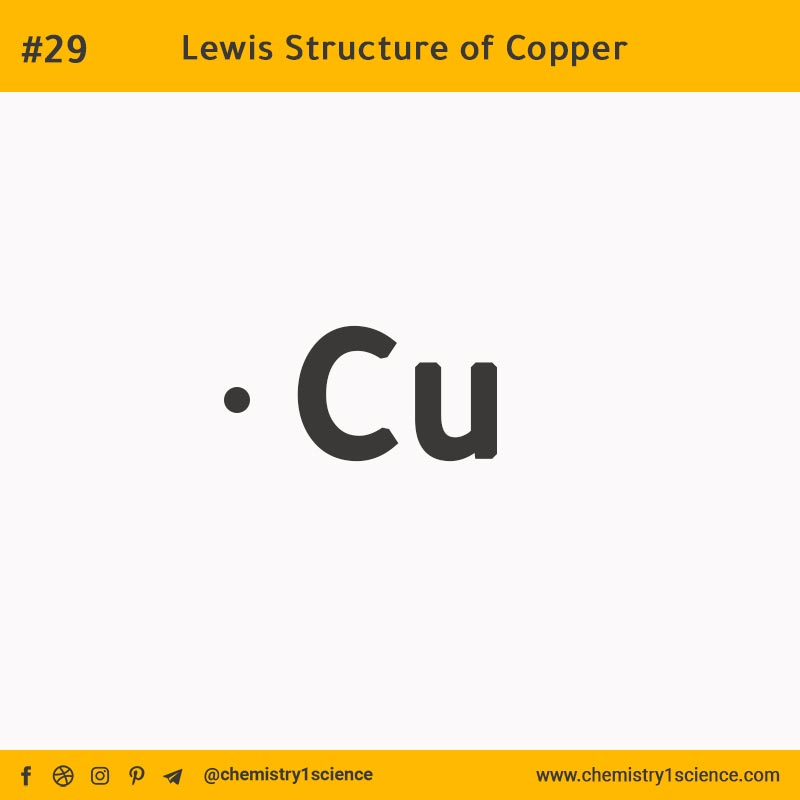
A Cu Lewis dot structure represents the valence electrons of copper atoms in a molecule or ion using dots around the element symbol, illustrating how electrons are shared or transferred during bonding.
Unlike main-group elements, copper (Cu) possesses a partially filled d-subshell (with 4s¹4d¹⁰ configuration), which complicates its bonding behavior. The dot structure simplifies this complexity by focusing on electron distribution patterns, helping chemists visualize bonding tendencies despite the metal’s tendency toward variable oxidation states.
Each dot signifies an unpaired or paired electron in the outermost shell, and arrangements reflect resonance forms when multiple stable configurations exist. For copper in its neutral atomic form, the unpaired 4s and 4d electrons contribute to its signature paramagnetic properties and realization in structures such as \[CuH2\]+ or complex ions like \[CuCl4]2−.
The Role of d-Electrons in Copper’s Bonding
Copper’s electronic configuration—[Ar] 3d¹⁰ 4s¹—means its valence electrons are primarily in the 4s orbital, with an extra 4d electron.
This d-electron richness enables copper to form both ionic and covalent bonds, depending on the surrounding ligands. The Lewis structure captures how these electrons participate in bonding: metal atoms donate electron density to electron-deficient sites or share electrons via d-orbital hybridization.
In molecular complexes, Cu atoms often exhibit oxidation states of +1 or +2, with each altering the electron count and symmetry of the dot structure. For instance, Cu⁺ (d¹⁰ configuration) tends toward linear bonding, while Cu²⁺ (d⁹) may favor trigonal or square planar geometries, reflected visually in resonance-dominated structures.
This adaptability underscores why the cu Lewis dot structure remains indispensable in predicting coordination chemistry.
Visualizing Reactivity Patterns with Dot Structures
The power of the Cu Lewis dot structure lies in its ability to predict how copper compounds behave in different chemical environments. Each dot pattern reveals key insights into oxidation tendencies, coordination geometries, and redox behavior. For example, excess electrons in transition states are directly indicated by additional dots in resonance forms, highlighting sites prone to nucleophilic attack or metal-ligand bond scission.
Consider \[Cu(NH3)2\] in aqueous solution: the dot structure depicts Cu²⁺ (d⁹) surrounded by three ammonia molecules and one chloride ion, showing partial electron delocalization through d-resonance.
This electronic distribution stabilizes the complex but also restricts symmetry, enabling ligand exchange crucial in biological and industrial catalysis. Similarly, copper sulfide minerals—such as chalcopyrite (CuFeS₂)—reveal lattice bonding patterns through extended dot network representations, showing how d-electrons mediate strong cation-anion interactions.
Variability in ligand types—ranging from strong-field CO to weak-field Cl−—alters electron density maps. Strong-field ligands induce low-spin configurations, reflected in elongated or compressed bond angles visible in detailed dot-based ray diagrams, ultimately affecting catalytic efficiency in reactions like CO oxidation.
Real-World Applications Shaped by Cu Lewis Structures
The practical impact of understanding cu Lewis dot structures spans multiple high-tech domains.
In catalysis, precise knowledge of electron distribution enables design of more efficient copper-based catalysts used in the Haber-Bosch process (indirectly via ammonia intermediates) and in green chemistry for selective oxidation reactions.
Materials science leverages this framework to develop conductive polymers, plasmonic nanoparticles, and corrosion-resistant alloys. For instance, gold-copper bimetallic nanoparticles—engineered by analyzing Cu polarization in dot structures—show enhanced electronic mobility, critical in flexible electronics and photovoltaics.
Biological systems also rely on copper’s electronic architecture. Enzymes like cytochrome c oxidase depend on Cu²⁺’s precise electron transfer capabilities, guided by dot structure insights into ligand coordination and redox states.
This understanding fuels breakthroughs in biomedical imaging and targeted drug delivery.
The Enduring Legacy of the Cu Lewis Dot Structure
While quantum mechanical models offer deeper precision, the Cu Lewis dot structure remains a vital educational and analytical tool. Its ability to simplify complex electron behavior into accessible visuals empowers chemists, educators, and engineers alike. From predicting complex stability to guiding synthetic design, it continues to unlock the reactivity of copper in both industrial innovation and fundamental science.
As new materials and technologies emerge, the Cu Lewis dot structure endures not just as a diagram—but as a foundational lens through which the quantum world of metals becomes tangible, predictable, and transformative.



Related Post

Jamaica’s Sky at Ground: Unlocking the True Dimensions of the Island Nation

What Did Cccp Stand For? Unraveling the Legacy of a Cold War Powerhouse

Ebony Ingot Id Skyrim: The Hidden Mystique Behind a Trader’s Most Coveted Currency

Honoring Life with Dignity: Pugh Funeral Home Serves Asheboro’s Community with Compassion and Care

