Unveiling PCl₃: The Critical Geometry and Electron Dynamics of Phosphorus Trichloride Lewis Structure
Unveiling PCl₃: The Critical Geometry and Electron Dynamics of Phosphorus Trichloride Lewis Structure
Phosphorus trichloride (PCl₃) stands as a vital compound in inorganic chemistry, serving as a cornerstone in industrial synthesis, chemical manufacturing, and educational chemistry. Its Lewis dot structure reveals fundamental insights into its molecular geometry, bonding behavior, and reactivity—elements that dictate how it interacts with other substances. By analyzing PCl₃’s Lewis configuration, chemists unlock explanations behind its trigonal pyramidal shape, polarity, and disproportionate chemical role in stacking reactions.
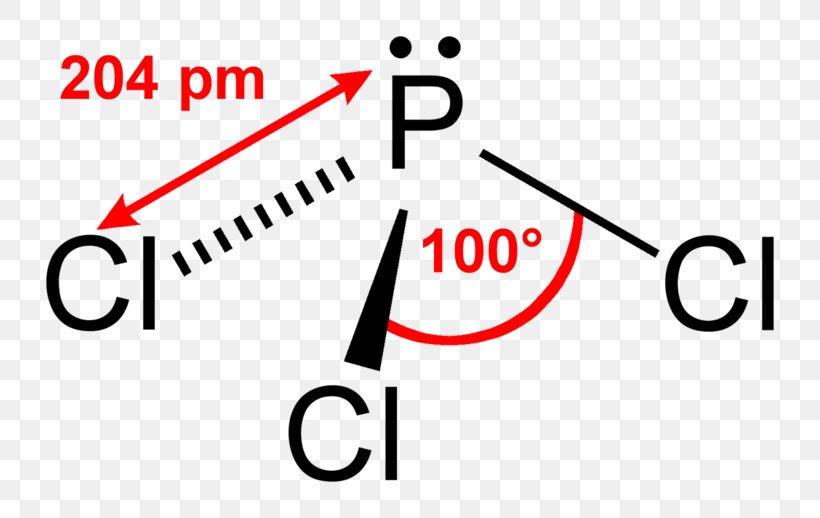
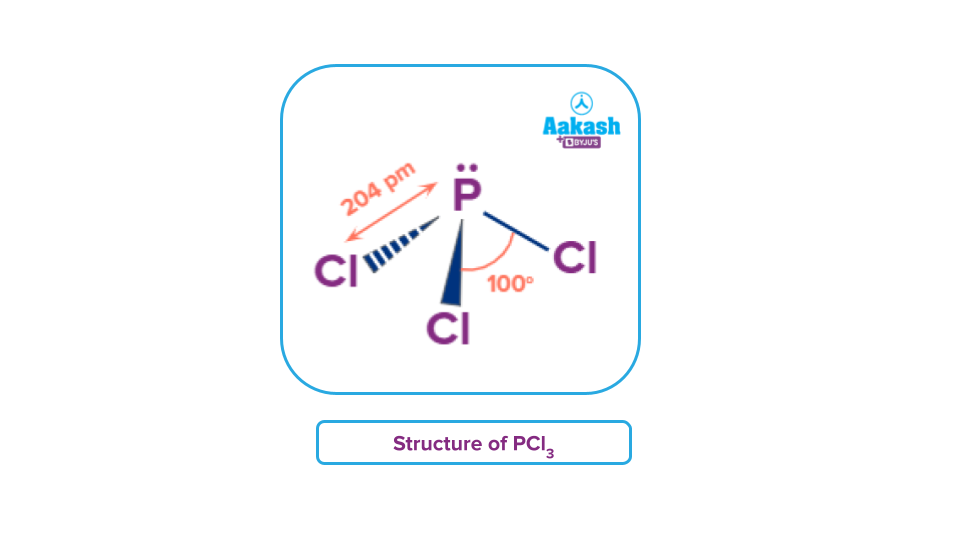
At the heart of PCl₃’s structure lies phosphorus center bonded to three chlorine atoms, with one lone pair of electrons reshaping the idealized molecular form.
This arrangement—governed by VSEPR theory—explains why the molecule departs from a perfect trigonal planar layout, adopting a distinct pyramidal geometry that profoundly influences its reactivity and physical properties.
The Molecular Blueprint: Breaking Down PCl₃ Lewis Representation
To visualize PCl₃, chemistry draws upon Lewis dot structures—simplified yet powerful diagrams that map valence electrons around central atoms. In PCl₃, phosphorus contributes five valence electrons, while each chlorine atom contributes seven. Each P–Cl bond forms from a shared pair, totaling six bonding pairs, yet the fourth electron remains localized as a lone pair.
This configuration leads to five electron domains around phosphorus, limiting its electron domain geometry to trigonal bipyramidal—Still, only three chlorine substitutions result, yielding the characteristic trigonal pyramidal shape.
Electron Count and Distribution: - Total valence electrons: 5 (P) + 3 × 7 (Cl) = 26 - Shared electrons in bonds: 6 × 2 = 12 - Remaining electron count (lone pair): 26 – 12 = 14 electrons → 7 pairs - Of these, three are used in bonds; seven remain – one pair at bond tip, six distributed as three lone pairs attached to phosphorus. - The lone pair repels bonding pairs, compressing C–Cl angles to approximately 100°, slightly less than ideal 107.5° in pure trigonal pyramidal models.This asymmetry influences not just shape, but also electrostatic distribution.
The lone pair introduces partial negative charge on phosphorus’ side, while chlorine atoms bear partial positive charges—making PCl₃ a weakly polar molecule despite its symmetry-truncated geometry. This polarity, subtle yet vital, underpins solubility trends and intermolecular interactions in liquid and vapor phases.
Geometric Nuances: From Theory to Real-World Structure
While the VSEPR model predicts trigonal pyramidal geometry, real-world PCl₃ molecules exhibit slight deviations. Experimental data from spectral analysis and X-ray crystallography reveal bond angles averaging 99–102°, consistent with bond strain from lone pair repulsion.
Unlike ammonia (NH₃), where lone pair repulsion is moderate, PCl₃’s larger central atom (phosphorus, atomic radius ~111 pm versus nitrogen’s ~71 pm) experiences lesser compression but stronger lone-pair effects due to expanded octet availability.
The spatial orientation of lone pairs also influences molecular symmetry. The lone pair occupies an equatorial position in the trigonal bipyramidal electron domain, maximizing separation from bonding pairs—a configuration known to reduce electron repulsion and stabilize the molecule. This orientation minimizes angular strain but limits rotational freedom, fixing the molecular geometry despite dynamic environments.
Bonding Character and Polarity: The Dipole Behind the Dipole Moment
Bond polarization

Related Post

The Inside Story Behind Jeff Verszyla’s Salary: What Sets His Compensation at the Cutting Edge

BinomialNomenclature of a Dog: Decoding Canine Precision and Identity

2009 Harley Electra Glide Classic: Performance, Features, and Troubleshooting the Gold Standard of Cruisers

Bruno Mars’ Billionaire Journey: From Humble Beginnings to Global Music Mogul

