What Charge Does a Neutron Have? The Neutral Particle That Shapes the Atom
What Charge Does a Neutron Have? The Neutral Particle That Shapes the Atom
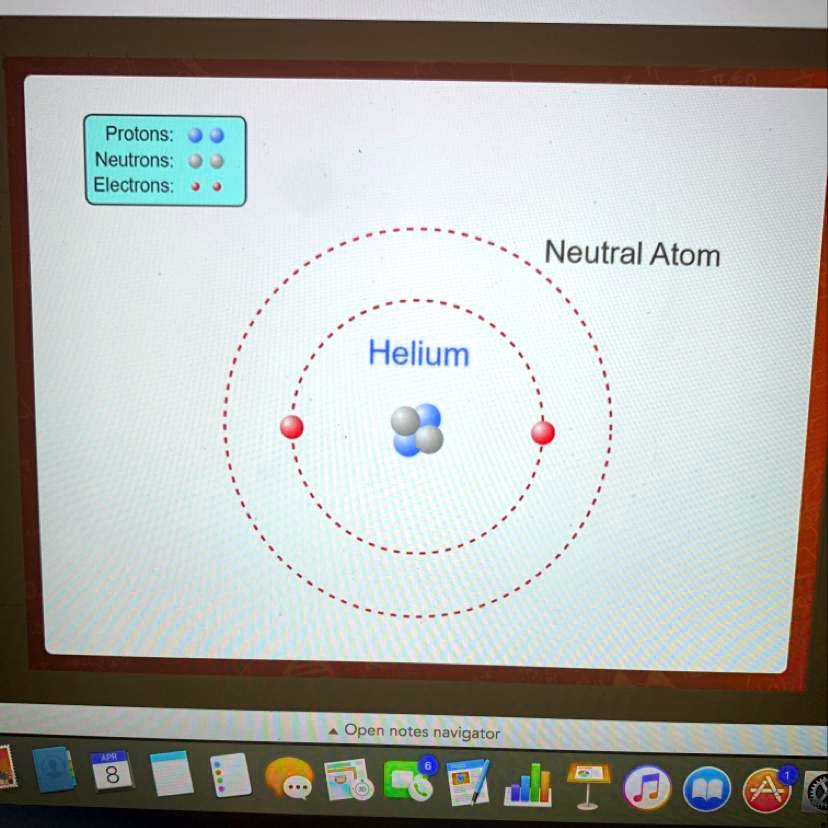
In the intricate world of atomic structure, one particle stands out for its invisible yet foundational role: the neutron. While protons carry a definitive positive charge and electrons bear a well-known negative charge, neutrons exist in a neutral limbo—carrying no electric charge at all. This subtle yet profound characteristic underpins atomic stability and enables the rich diversity of elements in the universe.
Understanding what charge a neutron has—not a trivial detail, but a cornerstone of modern physics—reveals why even neutral particles can wield such powerful influence over matter. Neutrons possess zero electric charge, a fact confirmed through decades of high-precision experiments and theoretical modeling. This neutrality arises from their internal composition: a neutron is made up of three fundamental quarks—one up quark and two down quarks—bound together by the strong nuclear force.
Unlike protons, which consist of two up quarks and one down quark, neutrons have two down quarks (each carrying -1/3 charge) and one up quark (+2/3), summing to a total charge of:
Charge = (-1/3) + (-1/3) + (+2/3) = 0This mathematical balance ensures the neutron’s neutrality, even though each constituent quark carries a fractional charge.
“The near-zero charge of the neutron might seem like a minor quirk,”says Dr. Elena Marquez, physicist at CERN’s particle physics division. “But it’s precisely this balance that allows stability in atomic nuclei, as protons alone would cause repulsion and disrupt structure.” This charge neutrality allows neutrons to exist in atomic nuclei without triggering Coulombic repulsion, enabling them to bond with protons under the pull of the strong force.
Without neutrons, most nuclei would be unstable or nonexistent—leading to a universe vastly different from the one observed. Quarks, Forces, and the Absence of Charge
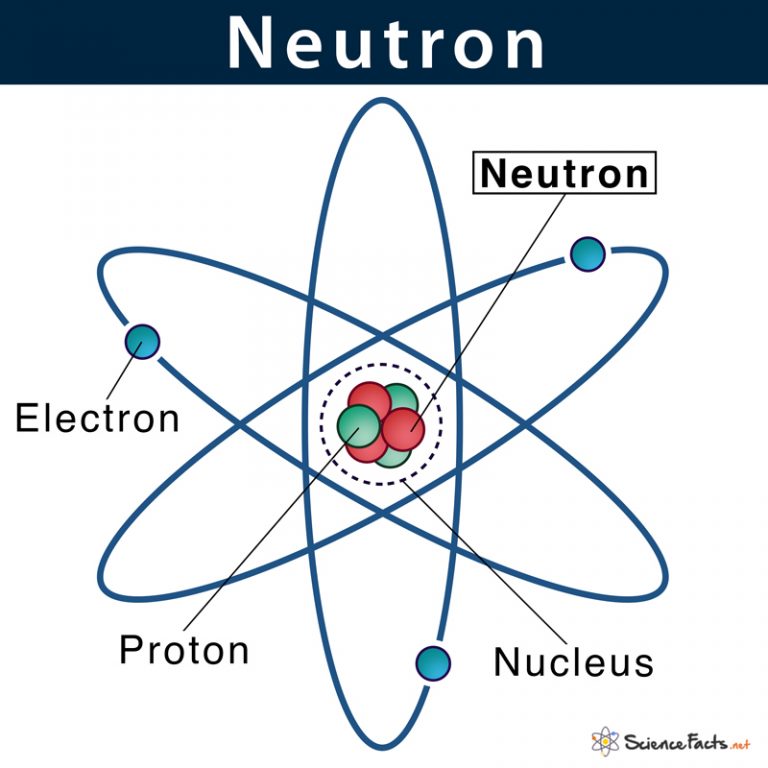
Neutrons, though electrically neutral, are not “nothing”—they are complex composite particles governed by quantum chromodynamics. Each neutron consists of three quarks held together by gluons, mediators of the strong nuclear force.
This force, far stronger than electromagnetism at subatomic scales, overcomes the electrostatic repulsion between positively charged protons in the nucleus. The neutron’s lack of charge means it contributes mass and nuclear binding without disrupting electrostatic equilibrium. Student studies often focus on charge to explain atomic behavior, but neutron neutrality offers a critical counterpoint: stability in matter doesn’t require instant charge balance.
“People assume only charged particles interact meaningfully,” explains Dr. Marquez. “But neutral neutrons demonstrate that internal structure and force balance are equally, if not more, vital.”
What makes neutrons uniquely powerful is their role as charge-neutral glue in nuclei.
While protons ensure identity and isotopic variation through charge, neutrons provide structural continuity. In isotopes like carbon-12 and carbon-14, the neutron count differentiates elements without altering charge, underscoring how neutrality enables diversity within atomic families.For instance, carbon-12 (six protons, six neutrons) contrasts sharply with carbon-14 (six protons, eight neutrons), yet both remain chemically defined by proton count and electron configuration. External comparisons often highlight neutron neutrality’s rarity. Most sub

Related Post

Exploring The Life And Career Of Jaden Boreanaz: A Rising Star Rising Above the Spotlight

Yaya Dacosta: Biography, Family, and Life Story of a Multifaceted Public Figure — Age, Husband, and the Son Who Shapes Her World
4Koy Com: The Silent Revolution Transforming Modern Business Operations

Meta Quest 2 vs 3 vs 3S: The Definitive Breakdown of Performance, Features, and Value

